Engineering protein nanocages as carriers for biomedical applications
- PMID: 32218880
- PMCID: PMC7091667
- DOI: 10.1038/am.2016.128
Engineering protein nanocages as carriers for biomedical applications
Abstract
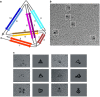
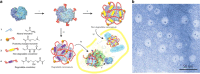
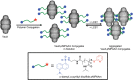
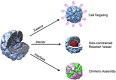
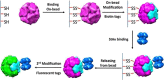
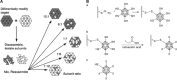
Protein nanocages have been explored as potential carriers in biomedicine. Formed by the self-assembly of protein subunits, the caged structure has three surfaces that can be engineered: the interior, the exterior and the intersubunit. Therapeutic and diagnostic molecules have been loaded in the interior of nanocages, while their external surfaces have been engineered to enhance their biocompatibility and targeting abilities. Modifications of the intersubunit interactions have been shown to modulate the self-assembly profile with implications for tuning the molecular release. We review natural and synthetic protein nanocages that have been modified using chemical and genetic engineering techniques to impart non-natural functions that are responsive to the complex cellular microenvironment of malignant cells while delivering molecular cargos with improved efficiencies and minimal toxicity.
Keywords: Biomaterials - proteins.
© The Author(s) 2017.
Figures








References
-
- Ferrer-Miralles N, Rodríguez-Carmona E, Corchero JL, García-Fruitós E, Vázquez E, Villaverde A. Engineering protein self-assembling in protein-based nanomedicines for drug delivery and gene therapy. Crit. Rev. Biotechnol. 2013;35:209–221. - PubMed
-
- Yan M, Du J, Gu Z, Liang M, Hu Y, Zhang W, Priceman S, Wu L, Hong Z, Zhou H, Liu Z, Segura T, Tang Y, Lu Y. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat. Nanotechnol. 2010;5:48–53. - PubMed
-
- Lee LA, Wang Q. Adaptations of nanoscale viruses and other protein cages for medical applications. Nanomedicine. 2006;2:137–149. - PubMed
-
- Flenniken ML, Uchida M, Liepold LO, Kang S, Young MJ, Douglas T. A library of protein cage architectures as nanomaterials. Curr. Top. Microbiol. 2009;327:71–93. - PubMed
-
- Uchida M, Klem MT, Allen M, Suci P, Flenniken M, Gillitzer E, Carpness Z, Liepold LO, Young M, Douglas T. Biological containers: protein cages as multifunctional nanoplatforms. Adv. Mater. 2007;19:1025–1042.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
