Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes
- PMID: 32219386
- PMCID: PMC7157181
- DOI: 10.1001/jama.2020.1906
Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes
Erratum in
-
Errors in Results.JAMA. 2021 Apr 6;325(13):1335. doi: 10.1001/jama.2021.2802. JAMA. 2021. PMID: 33821919 Free PMC article. No abstract available.
Abstract
Importance: Additional treatments are needed for heart failure with reduced ejection fraction (HFrEF). Sodium-glucose cotransporter 2 (SGLT2) inhibitors may be an effective treatment for patients with HFrEF, even those without diabetes.
Objective: To evaluate the effects of dapagliflozin in patients with HFrEF with and without diabetes.
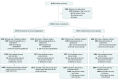
Design, setting, and participants: Exploratory analysis of a phase 3 randomized trial conducted at 410 sites in 20 countries. Patients with New York Heart Association classification II to IV with an ejection fraction less than or equal to 40% and elevated plasma N-terminal pro B-type natriuretic peptide were enrolled between February 15, 2017, and August 17, 2018, with final follow-up on June 6, 2019.
Interventions: Addition of once-daily 10 mg of dapagliflozin or placebo to recommended therapy.
Main outcomes and measures: The primary outcome was the composite of an episode of worsening heart failure or cardiovascular death. This outcome was analyzed by baseline diabetes status and, in patients without diabetes, by glycated hemoglobin level less than 5.7% vs greater than or equal to 5.7%.
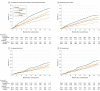
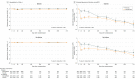
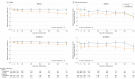
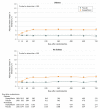
Results: Among 4744 patients randomized (mean age, 66 years; 1109 [23%] women; 2605 [55%] without diabetes), 4742 completed the trial. Among participants without diabetes, the primary outcome occurred in 171 of 1298 (13.2%) in the dapagliflozin group and 231 of 1307 (17.7%) in the placebo group (hazard ratio, 0.73 [95% CI, 0.60-0.88]). In patients with diabetes, the primary outcome occurred in 215 of 1075 (20.0%) in the dapagliflozin group and 271 of 1064 (25.5%) in the placebo group (hazard ratio, 0.75 [95% CI, 0.63-0.90]) (P value for interaction = .80). Among patients without diabetes and a glycated hemoglobin level less than 5.7%, the primary outcome occurred in 53 of 438 patients (12.1%) in the dapagliflozin group and 71 of 419 (16.9%) in the placebo group (hazard ratio, 0.67 [95% CI, 0.47-0.96]). In patients with a glycated hemoglobin of at least 5.7%, the primary outcome occurred in 118 of 860 patients (13.7%) in the dapagliflozin group and 160 of 888 (18.0%) in the placebo group (hazard ratio, 0.74 [95% CI, 0.59-0.94]) (P value for interaction = .72). Volume depletion was reported as an adverse event in 7.3% of patients in the dapagliflozin group and 6.1% in the placebo group among patients without diabetes and in 7.8% of patients in the dapagliflozin group and 7.8% in the placebo group among patients with diabetes. A kidney adverse event was reported in 4.8% of patients in the dapagliflozin group and 6.0% in the placebo group among patients without diabetes and in 8.5% of patients in the dapagliflozin group and 8.7% in the placebo group among patients with diabetes.
Conclusions and relevance: In this exploratory analysis of a randomized trial of patients with HFrEF, dapagliflozin compared with placebo, when added to recommended therapy, significantly reduced the risk of worsening heart failure or cardiovascular death independently of diabetes status.
Trial registration: ClinicalTrials.gov Identifier: NCT03036124.
Conflict of interest statement
Figures

Comment in
-
A Novel Cardioprotective Therapy That Also Improves Glycemia.JAMA. 2020 Apr 14;323(14):1349-1350. doi: 10.1001/jama.2020.1905. JAMA. 2020. PMID: 32219358 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

