Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial
- PMID: 32219442
- PMCID: PMC7290090
- DOI: 10.1182/blood.2020004856
Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial
Abstract
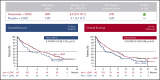
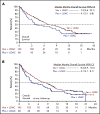
Effective treatment options are limited for patients with acute myeloid leukemia (AML) who cannot tolerate intensive chemotherapy. Adults age ≥18 years with newly diagnosed AML ineligible for intensive chemotherapy were enrolled in this international phase 3 randomized double-blind placebo-controlled trial. Patients (N = 211) were randomized 2:1 to venetoclax (n = 143) or placebo (n = 68) in 28-day cycles, plus low-dose cytarabine (LDAC) on days 1 to 10. Primary end point was overall survival (OS); secondary end points included response rate, transfusion independence, and event-free survival. Median age was 76 years (range, 36-93 years), 38% had secondary AML, and 20% had received prior hypomethylating agent treatment. Planned primary analysis showed a 25% reduction in risk of death with venetoclax plus LDAC vs LDAC alone (hazard ratio [HR], 0.75; 95% confidence interval [CI], 0.52-1.07; P = .11), although not statistically significant; median OS was 7.2 vs 4.1 months, respectively. Unplanned analysis with additional 6-month follow-up demonstrated median OS of 8.4 months for the venetoclax arm (HR, 0.70; 95% CI, 0.50-0.98; P = .04). Complete remission (CR) plus CR with incomplete blood count recovery rates were 48% and 13% for venetoclax plus LDAC and LDAC alone, respectively. Key grade ≥3 adverse events (venetoclax vs LDAC alone) were febrile neutropenia (32% vs 29%), neutropenia (47% vs 16%), and thrombocytopenia (45% vs 37%). Venetoclax plus LDAC demonstrates clinically meaningful improvement in remission rate and OS vs LDAC alone, with a manageable safety profile. Results confirm venetoclax plus LDAC as an important frontline treatment for AML patients unfit for intensive chemotherapy. This trial was registered at www.clinicaltrials.gov as #NCT03069352.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.H.W. has been a consultant for AbbVie, Celgene, Roche, Janssen, Astellas, Novartis, Amgen, MacroGenics, and Servier; has received research funding from AbbVie, Novartis, Celgene, and Servier; and is a former employee of the Walter and Eliza Hall Institute of Medical Research, which receives royalties in relation to venetoclax, and A.H.W. is entitled to a fraction of these payments. P.M. has received grants/research support from Astellas, Celgene, Daiichi Sankyo, Janssen, Karyopharm, Novartis, Pfizer, and Teva; has been a speaker/advisor for AbbVie, Celgene, Daiichi Sankyo, Incyte, Janssen, Karyopharm, Novartis, Pfizer, Teva, and Tolero; and has been a consultant for Agios, Astellas, Celgene, Daiichi Sankyo, Oryzon, and Tolero. V.I., I.K., D.A.S., O.S., Y.H., J.-Z.H., and A.M. have been investigators in AbbVie-sponsored clinical trials. C.D.D. has received grants/research support from AbbVie, Agios, Bayer, Celgene, and MedImmune and been a consultant for Agios. J.N. has been a consultant/advisor for Novartis, Roche, Pfizer, Amgen, and Takeda and received travel expenses from Amgen and Janssen. K.L. has received grants/research support from AbbVie, Novartis, Takeda, Roche, and Sandoz and received honoraria or speaker’s bureau/personal fees from AbbVie, Novartis, Takeda, Roche, Sandoz, Celgene, Jansen, and Amgen. I.K. has been an investigator in AbbVie-sponsored clinical trials. W.F. has served on the board of directors/advisory board for Amgen, ARIAD/Incyte, Pfizer, Novartis, Jazz Pharmaceuticals, and Celgene; received patents/royalties from Amgen; received support for meeting attendance from Amgen, Gilead, Jazz Pharmaceuticals, Servier, and Daiichi Sankyo; and received research funding from Amgen and Pfizer; M.P. has been a speaker/advisor for AbbVie, Amgen, Astellas, Genesis, Janssen, Novartis, and Pfizer. A.A. has received institutional research support (clinical trials) from AbbVie, Celgene, Sanofi, Takeda, Amgen, Pharmacyclics, Acerta, and Novartis. J.B. has been a consultant for Pfizer, Jazz, Novartis, Amgen, and Astellas and received travel expenses from Amgen and Novartis. V.M. has provided conference support attendance for Abbvie, Celgene, Novartis, Takeda, and Janssen and been a consultant for Celgene, Novartis, and Janssen. T.Y. has received research support/honoraria or been an advisor for Pfizer, Otsuka, Takeda, Astellas, Solasia, Gilead Sciences, and SymBio. P.P. has received grants/research support from AbbVie, Roche, Novartis, and Genesis and honoraria from AbbVie, Janssen, Roche, Novartis, Gilead, and Genesis. B.C., S.G., Q.J., W.M., and J.H. are employees of AbbVie and may hold stock or have stock options.
Figures
Comment in
-
The long road: improving outcome in elderly "unfit" AML?Blood. 2020 Jun 11;135(24):2114-2115. doi: 10.1182/blood.2020005651. Blood. 2020. PMID: 32526019 No abstract available.
References
-
- Juliusson G, Antunovic P, Derolf A, et al. . Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179-4187. - PubMed
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program: Cancer stat facts: leukemia—acute myeloid leukemia. Available at: https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 3 September 2019.
-
- Amadori S, Suciu S, Selleslag D, et al. . Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979. - PubMed
-
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. . Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. - PMC - PubMed

