Calreticulin haploinsufficiency augments stem cell activity and is required for onset of myeloproliferative neoplasms in mice
- PMID: 32219445
- PMCID: PMC7332892
- DOI: 10.1182/blood.2019003358
Calreticulin haploinsufficiency augments stem cell activity and is required for onset of myeloproliferative neoplasms in mice
Abstract
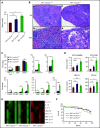
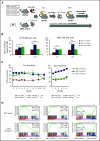
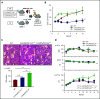
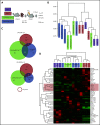
Mutations in JAK2, myeloproliferative leukemia virus (MPL), or calreticulin (CALR) occur in hematopoietic stem cells (HSCs) and are detected in more than 80% of patients with myeloproliferative neoplasms (MPNs). They are thought to play a driver role in MPN pathogenesis via autosomal activation of the JAK-STAT signaling cascade. Mutant CALR binds to MPL, activates downstream MPL signaling cascades, and induces essential thrombocythemia in mice. However, embryonic lethality of Calr-deficient mice precludes determination of a role for CALR in hematopoiesis. To clarify the role of CALR in normal hematopoiesis and MPN pathogenesis, we generated hematopoietic cell-specific Calr-deficient mice. CALR deficiency had little effect on the leukocyte count, hemoglobin levels, or platelet count in peripheral blood. However, Calr-deficient mice showed some hematopoietic properties of MPN, including decreased erythropoiesis and increased myeloid progenitor cells in the bone marrow and extramedullary hematopoiesis in the spleen. Transplantation experiments revealed that Calr haploinsufficiency promoted the self-renewal capacity of HSCs. We generated CALRdel52 mutant transgenic mice with Calr haploinsufficiency as a model that mimics human MPN patients and found that Calr haploinsufficiency restored the self-renewal capacity of HSCs damaged by CALR mutations. Only recipient mice transplanted with Lineage-Sca1+c-kit+ cells harboring both CALR mutation and Calr haploinsufficiency developed MPN in competitive conditions, showing that CALR haploinsufficiency was necessary for the onset of CALR-mutated MPNs.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing interests.
Figures




Comment in
-
Mutant CALR functions: gains and losses.Blood. 2020 Jul 2;136(1):6-7. doi: 10.1182/blood.2020005805. Blood. 2020. PMID: 32614958 No abstract available.
References
-
- Swerdlow SHCE, Harris NL, Jaffe ES, et al. . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised Fourth Edition Geneva, Switzerland: IARC press; 2017.
-
- Kralovics R, Passamonti F, Buser AS, et al. . A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779-1790. - PubMed
-
- James C, Ugo V, Le Couédic JP, et al. . A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144-1148. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, et al. ; Cancer Genome Project . Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054-1061. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. . Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387-397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

