ATPase Domain AFG3L2 Mutations Alter OPA1 Processing and Cause Optic Neuropathy
- PMID: 32219868
- PMCID: PMC7383914
- DOI: 10.1002/ana.25723
ATPase Domain AFG3L2 Mutations Alter OPA1 Processing and Cause Optic Neuropathy
Abstract
Objective: Dominant optic atrophy (DOA) is the most common inherited optic neuropathy, with a prevalence of 1:12,000 to 1:25,000. OPA1 mutations are found in 70% of DOA patients, with a significant number remaining undiagnosed.
Methods: We screened 286 index cases presenting optic atrophy, negative for OPA1 mutations, by targeted next generation sequencing or whole exome sequencing. Pathogenicity and molecular mechanisms of the identified variants were studied in yeast and patient-derived fibroblasts.
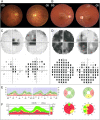
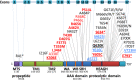
Results: Twelve cases (4%) were found to carry novel variants in AFG3L2, a gene that has been associated with autosomal dominant spinocerebellar ataxia 28 (SCA28). Half of cases were familial with a dominant inheritance, whereas the others were sporadic, including de novo mutations. Biallelic mutations were found in 3 probands with severe syndromic optic neuropathy, acting as recessive or phenotype-modifier variants. All the DOA-associated AFG3L2 mutations were clustered in the ATPase domain, whereas SCA28-associated mutations mostly affect the proteolytic domain. The pathogenic role of DOA-associated AFG3L2 mutations was confirmed in yeast, unraveling a mechanism distinct from that of SCA28-associated AFG3L2 mutations. Patients' fibroblasts showed abnormal OPA1 processing, with accumulation of the fission-inducing short forms leading to mitochondrial network fragmentation, not observed in SCA28 patients' cells.
Interpretation: This study demonstrates that mutations in AFG3L2 are a relevant cause of optic neuropathy, broadening the spectrum of clinical manifestations and genetic mechanisms associated with AFG3L2 mutations, and underscores the pivotal role of OPA1 and its processing in the pathogenesis of DOA. ANN NEUROL 2020 ANN NEUROL 2020;88:18-32.
© 2020 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures




References
-
- Carelli V, Ross‐Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 2004;23:53–89. - PubMed
-
- Alexander C, Votruba M, Pesch UE, et al. OPA1, encoding a dynamin‐related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 2000;26:211–215. - PubMed
-
- Delettre C, Lenaers G, Griffoin JM, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin‐related protein, is mutated in dominant optic atrophy. Nat Genet 2000;26:207–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

