Intestinal fatty acid-binding protein mediates atherosclerotic progress through increasing intestinal inflammation and permeability
- PMID: 32220004
- PMCID: PMC7205806
- DOI: 10.1111/jcmm.15173
Intestinal fatty acid-binding protein mediates atherosclerotic progress through increasing intestinal inflammation and permeability
Retraction in
-
Retraction.J Cell Mol Med. 2022 Feb;26(3):955. doi: 10.1111/jcmm.17151. Epub 2022 Jan 10. J Cell Mol Med. 2022. PMID: 35001505 Free PMC article. No abstract available.
Abstract
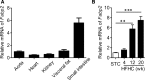
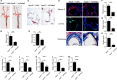
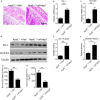
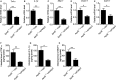
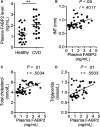
Atherosclerosis is one of leading phenotypes of cardiovascular diseases, featured with increased vascular intima-media thickness (IMT) and unstable plaques. The interaction between gastrointestinal system and cardiovascular homeostasis is emerging as a hot topic. Therefore, the present study aimed to explore the role of an intestinal protein, intestinal fatty acid-binding protein (I-FABP/FABP2) in the atherosclerotic progress. In western diet-fed ApoE-/- mice, FABP2 was highly expressed in intestine. Silence of intestinal Fabp2 attenuated western diet-induced atherosclerotic phenotypes, including decreasing toxic lipid accumulation, vascular fibrosis and inflammatory response. Mechanistically, intestinal Fabp2 knockdown improved intestinal permeability through increasing the expression of tight junction proteins. Meanwhile, intestinal Fabp2 knockdown mice exhibited down-regulation of intestinal inflammation in western diet-fed ApoE-/- mice. In clinical patients, the circulating level of FABP2 was obviously increased in patients with cardiovascular disease and positively correlated with the value of carotid intima-media thickness, total cholesterol and triglyceride. In conclusion, FABP2-induced intestinal permeability could address a potential role of gastrointestinal system in the development of atherosclerosis, and targeting on intestinal FABP2 might provide a therapeutic approach to protect against atherosclerosis.
Keywords: atherosclerosis; inflammation; intestinal fatty acid-binding protein; intestinal permeability.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Sivelestat Alleviates Atherosclerosis by Improving Intestinal Barrier Function and Reducing Endotoxemia.Front Pharmacol. 2022 Apr 4;13:838688. doi: 10.3389/fphar.2022.838688. eCollection 2022. Front Pharmacol. 2022. PMID: 35444551 Free PMC article.
-
Medium-chain triglycerides promote macrophage reverse cholesterol transport and improve atherosclerosis in ApoE-deficient mice fed a high-fat diet.Nutr Res. 2016 Sep;36(9):964-973. doi: 10.1016/j.nutres.2016.06.004. Epub 2016 Jun 4. Nutr Res. 2016. PMID: 27632916
-
Chronic intermittent hypoxia exposure induces atherosclerosis in ApoE knockout mice: role of NF-κB p50.Am J Pathol. 2012 Nov;181(5):1530-9. doi: 10.1016/j.ajpath.2012.07.024. Epub 2012 Aug 30. Am J Pathol. 2012. PMID: 22940439
-
Intestinal fatty acid binding protein: A rising therapeutic target in lipid metabolism.Prog Lipid Res. 2022 Jul;87:101178. doi: 10.1016/j.plipres.2022.101178. Epub 2022 Jun 30. Prog Lipid Res. 2022. PMID: 35780915 Review.
-
Discovering Inflammation in Atherosclerosis: Insights from Pathogenic Pathways to Clinical Practice.Int J Mol Sci. 2024 May 30;25(11):6016. doi: 10.3390/ijms25116016. Int J Mol Sci. 2024. PMID: 38892201 Free PMC article. Review.
Cited by
-
Piperine Improves Obesity by Inhibiting Fatty Acid Absorption and Repairing Intestinal Barrier Function.Plant Foods Hum Nutr. 2021 Dec;76(4):410-418. doi: 10.1007/s11130-021-00919-2. Epub 2021 Sep 30. Plant Foods Hum Nutr. 2021. PMID: 34591253
-
Role and Mechanism of Gut Microbiota in Human Disease.Front Cell Infect Microbiol. 2021 Mar 17;11:625913. doi: 10.3389/fcimb.2021.625913. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33816335 Free PMC article. Review.
-
Integrated Transcriptome and 16S rDNA Analyses Reveal That Transport Stress Induces Oxidative Stress and Immune and Metabolic Disorders in the Intestine of Hybrid Yellow Catfish (Tachysurus fulvidraco♀ × Pseudobagrus vachellii♂).Antioxidants (Basel). 2022 Aug 31;11(9):1737. doi: 10.3390/antiox11091737. Antioxidants (Basel). 2022. PMID: 36139809 Free PMC article.
-
A study on the treatment effects of Crataegus pinnatifida polysaccharide on non-alcoholic fatty liver in mice by modulating gut microbiota.Front Vet Sci. 2024 Mar 27;11:1383801. doi: 10.3389/fvets.2024.1383801. eCollection 2024. Front Vet Sci. 2024. PMID: 38601914 Free PMC article.
-
Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease.Am J Physiol Heart Circ Physiol. 2020 Dec 1;319(6):H1227-H1233. doi: 10.1152/ajpheart.00612.2020. Epub 2020 Sep 28. Am J Physiol Heart Circ Physiol. 2020. PMID: 32986965 Free PMC article. Review.
References
-
- Jonsson AL, Backhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79‐87. - PubMed
-
- Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761‐1772. - PubMed
-
- Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia‐Induced Inflammation in Apoe−/− Mice. Circulation. 2016;133:2434‐2446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

