Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy
- PMID: 32220256
- PMCID: PMC7102435
- DOI: 10.1186/s12967-020-02306-y
Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy
Abstract
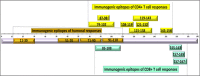
Introduction: Cancer Immunotherapy has recently emerged as a promising and effective modality to treat different malignancies. Antigenic profiling of cancer tissues and determination of any pre-existing immune responses to cancer antigens may help predict responses to immune intervention in cancer. NY-ESO-1, a cancer testis antigen is the most immunogenic antigen to date. The promise of NY-ESO-1 as a candidate for specific immune recognition of cancer comes from its restricted expression in normal adult tissue but frequent occurrence in multiple tumors including melanoma and carcinomas of lung, esophageal, liver, gastric, prostrate, ovarian, and bladder.
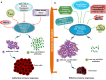
Main body: This review summarizes current knowledge of NY-ESO-1 as efficient biomarker and target of immunotherapy. It also addresses limitations and challenges preventing a robust immune response to NY-ESO-1 expressing cancers, and describes pre-clinical and clinical observations relevant to NY-ESO-1 immunity, holding potential therapeutic relevance for cancer treatment.
Conclusion: NY-ESO-1 induces strong immune responses in cancer patients but has limited objective clinical responses to NY-ESO-1 expressing tumors due to effect of competitive negative signaling from immune-checkpoints and immune-suppressive tumor microenvironment. We propose that combination therapy to increase the efficacy of NY-ESO-1 specific immunotherapeutic interventions should be explored to unleash the immune response against NY-ESO-1 expressing tumors.
Keywords: Cancer immunotherapy; Cancer testis antigen; Cancer vaccine; Immune checkpoint inhibitors; NY-ESO-1; Tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

