Bi-allelic Variants in the GPI Transamidase Subunit PIGK Cause a Neurodevelopmental Syndrome with Hypotonia, Cerebellar Atrophy, and Epilepsy
- PMID: 32220290
- PMCID: PMC7118585
- DOI: 10.1016/j.ajhg.2020.03.001
Bi-allelic Variants in the GPI Transamidase Subunit PIGK Cause a Neurodevelopmental Syndrome with Hypotonia, Cerebellar Atrophy, and Epilepsy
Abstract
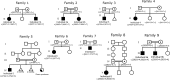
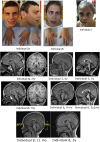
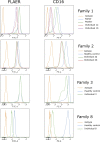
Glycosylphosphatidylinositol (GPI)-anchored proteins are critical for embryogenesis, neurogenesis, and cell signaling. Variants in several genes participating in GPI biosynthesis and processing lead to decreased cell surface presence of GPI-anchored proteins (GPI-APs) and cause inherited GPI deficiency disorders (IGDs). In this report, we describe 12 individuals from nine unrelated families with 10 different bi-allelic PIGK variants. PIGK encodes a component of the GPI transamidase complex, which attaches the GPI anchor to proteins. Clinical features found in most individuals include global developmental delay and/or intellectual disability, hypotonia, cerebellar ataxia, cerebellar atrophy, and facial dysmorphisms. The majority of the individuals have epilepsy. Two individuals have slightly decreased levels of serum alkaline phosphatase, while eight do not. Flow cytometric analysis of blood and fibroblasts from affected individuals showed decreased cell surface presence of GPI-APs. The overexpression of wild-type (WT) PIGK in fibroblasts rescued the levels of cell surface GPI-APs. In a knockout cell line, transfection with WT PIGK also rescued the GPI-AP levels, but transfection with the two tested mutant variants did not. Our study not only expands the clinical and known genetic spectrum of IGDs, but it also expands the genetic differential diagnosis for cerebellar atrophy. Given the fact that cerebellar atrophy is seen in other IGDs, flow cytometry for GPI-APs should be considered in the work-ups of individuals presenting this feature.
Keywords: GPI8; PIGK; glycosylphosphatidylinositol (GPI); inherited GPI deficiency disorders (IGDs); transamidase.
Copyright © 2020 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics. F.M.Z. and R.P. are employees of GeneDx.
Figures



References
-
- Ohishi K., Nagamune K., Maeda Y., Kinoshita T. Two subunits of glycosylphosphatidylinositol transamidase, GPI8 and PIG-T, form a functionally important intermolecular disulfide bridge. J. Biol. Chem. 2003;278:13959–13967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

