Analysis of Plasma Products for Cellular Contaminants: Comparing Standard Preparation Methods
- PMID: 32220451
- PMCID: PMC7682813
- DOI: 10.1016/j.jamcollsurg.2019.12.042
Analysis of Plasma Products for Cellular Contaminants: Comparing Standard Preparation Methods
Abstract
Background: Recent reports suggest that component plasma products contain significant quantities of cellular contamination. We hypothesized that leukoreduction of whole blood before preparation of derived plasma is an effective method to prevent cellular contamination of stored plasma.
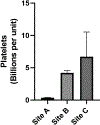
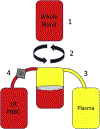
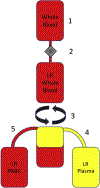
Study design: Samples of never-frozen liquid plasma prepared by standard methods (n = 25) were obtained from 3 regional blood centers that supply 3 major trauma centers. Samples were analyzed for leukocyte and platelet contamination by flow cytometry. To determine if leukoreduction of whole blood before centrifugation and expression of plasma prevents cellular contamination of liquid plasma, 1 site generated 6 additional units of liquid plasma from leukoreduced whole blood, which were then compared with units of liquid plasma derived by standard processing.
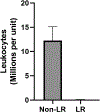
Results: Across all centers, each unit of never-frozen liquid plasma contained a mean of 12.8 ± 3.0 million leukocytes and a mean of 4.6 ± 2 billion platelets. Introduction of whole blood leukoreduction (LR) before centrifugation and plasma extraction essentially eliminated all contaminating leukocytes (Non-LR: 12.3 ± 2.9 million vs LR: 0.05 ± 0.05 million leukocytes) and platelets (Non-LR: 4.2 ± 0.3 billion platelets vs LR: 0.00 ± 0.00 billion platelets).
Conclusions: Despite widespread belief that stored plasma is functionally acellular, testing of liquid plasma from 3 regional blood banks revealed a significant amount of previously unrecognized cellular contamination. Introduction of a leukoreduction step before whole blood centrifugation essentially eliminated detectable leukocyte and platelet contaminants from plasma. Therefore, our study highlights a straightforward and cost-effective method to eliminate cellular contamination of stored plasma.
Copyright © 2020. Published by Elsevier Inc.
Figures


Comment in
-
Discussion.J Am Coll Surg. 2020 Apr;230(4):602-604. doi: 10.1016/j.jamcollsurg.2020.02.005. J Am Coll Surg. 2020. PMID: 32220452 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

