Stiff-Stilbene Photoswitches: From Fundamental Studies to Emergent Applications
- PMID: 32222016
- PMCID: PMC7496324
- DOI: 10.1002/anie.202001031
Stiff-Stilbene Photoswitches: From Fundamental Studies to Emergent Applications
Abstract
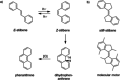
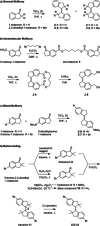
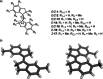
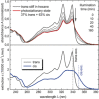
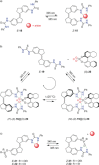
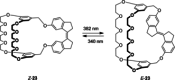
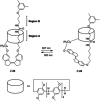
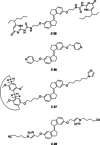
Stiff-stilbene, a sterically restricted fused ring analogue of stilbene, has been regularly used as a model compound in theoretical studies of stilbene photoisomerization. Lately, owing to its excellent photoswitching properties, it is increasingly being applied to reversibly control the properties and function of chemical as well as biological systems. Stiff-stilbene photoswitches possess a number of advantageous properties including a high quantum yield for photoisomerization and a high thermal stability. Furthermore, they undergo a large geometrical change upon isomerization and their synthesis is straightforward. Herein, we provide an overview of the basic properties of stiff-stilbene and of recent applications in supramolecular chemistry, catalysis, and biological systems.
Keywords: catalysis; molecular switches; photochromism; self-assembly; stiff-stilbene.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Push-Pull Stiff-Stilbene: Proton-Gated Visible-Light Photoswitching and Acid-Catalyzed Isomerization.Chemistry. 2021 Dec 9;27(69):17346-17350. doi: 10.1002/chem.202103052. Epub 2021 Oct 21. Chemistry. 2021. PMID: 34605565 Free PMC article.
-
Sterically Hindered Stiff-Stilbene Photoswitch Offers Large Motions, 90% Two-Way Photoisomerization, and High Thermal Stability.J Org Chem. 2022 Dec 2;87(23):15762-15770. doi: 10.1021/acs.joc.2c01566. Epub 2022 Nov 15. J Org Chem. 2022. PMID: 36378160
-
All-visible-light-driven stiff-stilbene photoswitches.Chem Sci. 2024 Mar 27;15(18):6763-6769. doi: 10.1039/d4sc00983e. eCollection 2024 May 8. Chem Sci. 2024. PMID: 38725493 Free PMC article.
-
Synthetic approaches toward stilbenes and their related structures.Mol Divers. 2017 May;21(2):483-509. doi: 10.1007/s11030-017-9736-9. Epub 2017 Apr 21. Mol Divers. 2017. PMID: 28429182 Free PMC article. Review.
-
Multiple Azoarenes Based Systems - Photoswitching, Supramolecular Chemistry and Application Prospects.Chem Rec. 2022 Nov;22(11):e202200074. doi: 10.1002/tcr.202200074. Epub 2022 Jul 21. Chem Rec. 2022. PMID: 35860915 Review.
Cited by
-
Red-light photoswitching of indigos in polymer thin films.Chem Sci. 2023 Feb 22;14(10):2482-2488. doi: 10.1039/d2sc06790k. eCollection 2023 Mar 8. Chem Sci. 2023. PMID: 36908950 Free PMC article.
-
Combined Photopolymerization and Localized Photochromism by Aza-Diarylethene and Hemiindigo Synergy.Adv Mater. 2025 Jan;37(3):e2411223. doi: 10.1002/adma.202411223. Epub 2024 Nov 21. Adv Mater. 2025. PMID: 39573834 Free PMC article.
-
Photoswitchable Binary Nanopore Conductance and Selective Electronic Detection of Single Biomolecules under Wavelength and Voltage Polarity Control.ACS Nano. 2022 Apr 26;16(4):5537-5544. doi: 10.1021/acsnano.1c10039. Epub 2022 Mar 14. ACS Nano. 2022. PMID: 35286058 Free PMC article.
-
Light and protonation-controlled complex formation between sulfate ions and a stiff-stilbene based bis(cyclopeptide).Chem Sci. 2025 Mar 24;16(18):7822-7828. doi: 10.1039/d5sc00766f. eCollection 2025 May 7. Chem Sci. 2025. PMID: 40177314 Free PMC article.
-
Azobenzene-Oxindole Photochromic Dyads.Angew Chem Int Ed Engl. 2025 May 26;64(22):e202501872. doi: 10.1002/anie.202501872. Epub 2025 Apr 2. Angew Chem Int Ed Engl. 2025. PMID: 40126028 Free PMC article.
References
-
- Lubbe A. S., van Leeuwen T., Wezenberg S. J., Feringa B. L., Tetrahedron 2017, 73, 4837–4848.
-
- Molecular Switches, 2nd ed. (Eds.: B. L. Feringa, W. R. Browne), Wiley-VCH, Weinheim, 2011.
-
- Russew M.-M., Hecht S., Adv. Mater. 2010, 22, 3348–3360. - PubMed
-
- Qu D.-H., Wang Q.-C., Zhang Q.-W., Ma X., Tian H., Chem. Rev. 2015, 115, 7543–7588. - PubMed
-
- Nie H., Self J. L., Kuenstler A. S., Hayward R. C., Read de Alaniz J., Adv. Opt. Mater. 2019, 7, 1900224.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

