Pharmacokinetics of polatuzumab vedotin in combination with R/G-CHP in patients with B-cell non-Hodgkin lymphoma
- PMID: 32222808
- PMCID: PMC7188703
- DOI: 10.1007/s00280-020-04054-8
Pharmacokinetics of polatuzumab vedotin in combination with R/G-CHP in patients with B-cell non-Hodgkin lymphoma
Abstract
Purpose: The phase Ib/II open-label study (NCT01992653) evaluated the antibody-drug conjugate polatuzumab vedotin (pola) plus rituximab/obinutuzumab, cyclophosphamide, doxorubicin, and prednisone (R/G-CHP) as first-line therapy for B-cell non-Hodgkin lymphoma (B-NHL). We report the pharmacokinetics (PK) and drug-drug interaction (DDI) for pola.
Methods: Six or eight cycles of pola 1.0-1.8 mg/kg were administered intravenously every 3 weeks (q3w) with R/G-CHP. Exposures of pola [including antibody-conjugated monomethyl auristatin E (acMMAE) and unconjugated MMAE] and R/G-CHP were assessed by non-compartmental analysis and/or descriptive statistics with cross-cycle comparisons to cycle 1 and/or after multiple cycles. Pola was evaluated as a potential victim and perpetrator of a PK drug-drug interaction with R/G-CHP. Population PK (popPK) analysis assessed the impact of prior treatment status (naïve vs. relapsed/refractory) on pola PK.
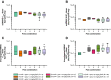
Results: Pola PK was similar between treatment arms and independent of line of therapy. Pola PK was dose proportional from 1.0 to 1.8 mg/kg with R/G-CHP. Geometric mean volume of distribution and clearance of acMMAE ranged from 57.3 to 95.6 mL/kg and 12.7 to 18.2 mL/kg/day, respectively. acMMAE exhibited multi-exponential decay (elimination half-life ~ 1 week). Unconjugated MMAE exhibited formation rate-limited kinetics. Exposures of pola with R/G-CHP were similar to those in the absence of CHP; exposures of R/G-CHP in the presence of pola were comparable to those in the absence of pola.
Conclusions: Pola PK was well characterized with no clinically meaningful DDIs with R/G-CHP. Findings are consistent with previous studies of pola + R/G, and support pola + R/G-CHP use in previously untreated diffuse large B-cell lymphoma.
Keywords: B-cell non-Hodgkin lymphoma; Combination therapy; Drug interactions; Pharmacokinetics; Phase Ib/II; Polatuzumab vedotin.
Conflict of interest statement
This study was sponsored by Genentech, Inc and F. Hoffmann-La Roche Ltd. All study authors are employees and stockholders of Genentech, Inc and F. Hoffmann-La Roche Ltd.
Figures

References
-
- Polson AG, Yu SF, Elkins K, Zheng B, Clark S, Ingle GS, Slaga DS, Giere L, Du C, Tan C, Hongo JA, Gogineni A, Cole MJ, Vandlen R, Stephan JP, Young J, Chang W, Scales SJ, Ross S, Eaton D, Ebens A. Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood. 2007;110:616–623. doi: 10.1182/blood-2007-01-066704. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

