A Three-Part, Randomised Study to Investigate the Safety, Tolerability, Pharmacokinetics and Mode of Action of BC 007, Neutraliser of Pathogenic Autoantibodies Against G-Protein Coupled Receptors in Healthy, Young and Elderly Subjects
- PMID: 32222912
- PMCID: PMC7181550
- DOI: 10.1007/s40261-020-00903-9
A Three-Part, Randomised Study to Investigate the Safety, Tolerability, Pharmacokinetics and Mode of Action of BC 007, Neutraliser of Pathogenic Autoantibodies Against G-Protein Coupled Receptors in Healthy, Young and Elderly Subjects
Abstract
Background and objective: BC 007 is a substance with a novel and innovative mode of action for the first-time causal treatment of chronic heart failure, associated with the occurrence of autoantibodies against the β1-adrenoceptor, and other diseases of mostly the heart and vascular system, being accompanied by the occurrence of functionally active agonistic autoantibodies against G-protein-coupled receptors (fGPCR-AAb). The proposed mechanism of action of BC 007 is the neutralisation of these pathogenic autoantibodies which stimulate the respective receptor. To evaluate the safety, tolerability, pharmacokinetics and mode of action of BC 007, single intravenous infusions of increasing concentration were given to healthy young males and healthy elderly autoantibody-negative and autoantibody-positive participants of both sexes.
Methods: This study was subdivided into three parts. Part A was a single-centre, randomised, double-blind, placebo-controlled safety and tolerability study including healthy young male autoantibody-negative Whites (N = 23) and Asians (N = 1), testing doses of 15, 50 and 150 mg BC 007 (Cohorts 1-3) and elderly male and female Whites (N = 8), testing a dose of 150 mg BC 007 (Cohort 4), randomly assigned in a 3:1 ratio to BC 007 or placebo. Open-label Part B included fGPCR-AAb-positive subjects (50 and 150 mg BC 007, Cohorts 1 and 2, respectively). Open-label Part C included fGPCR-AAb-positive subjects for testing doses of 300, 450, 750, 1350 mg and 1900 mg BC 007. Lower doses were either given as an infusion or divided into a bolus plus infusion up to a dose of 300 mg followed by a constant bolus of 150 mg up to a dose of 750 mg, while at doses of 1350 mg and 1900 mg it was a slow infusion with a constant infusion rate. Infusion times increased with increasing dose from 20 min (15, 50 or 150 mg) to 40 min (300, 450 or 750 mg), 75 min (1350 mg) and 105 min (1900 mg).
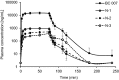
Results: The mean observed BC 007 area under the concentration-time curve (AUC0-24) increased with increasing dose in a dose proportional manner (slope estimate of 1.039). No serious adverse events were observed. Drug-related adverse events were predominantly the expected mild-to-moderate increase in bleeding time (aPTT), beginning with a dose of 50 mg, which paralleled the infusion and returned to normal shortly after infusion. fGPCR-AAb neutralisation efficiency increased with increasing dose and was achieved for all subjects in the last cohort.
Conclusion: BC 007 is demonstrated to be safe and well tolerated. BC 007 neutralised fGPCR-AAb, showing a trend for a dose-response relationship in elderly healthy but fGPCR-AAb-positive subjects. CLINICALTRIALS.
Gov registration number: NCT02955420.
Conflict of interest statement
Niels-Peter Becker, Annekathrin Haberland, Katrin Wenzel, Peter Göttel, Gerd Wallukat, Hanna Davideit, Sarah Schulze-Rothe, Anne-Sophie Hönicke, Ingolf Schimke, Sabine Bartel, Johannes Müller, Susanne Becker are employed at Berlin Cures GmbH. Annekathrin Haberland, Peter Göttel, Gerd Wallukat, Ingolf Schimke, Johannes Müller are shareholders of Berlin Cures Holding AG, the parent company of the Berliner branch Berlin Cures GmbH. All other authors have nothing to declare.
Figures





Similar articles
-
Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial.Lancet. 2015 Aug 15;386(9994):680-90. doi: 10.1016/S0140-6736(15)60732-2. Epub 2015 Jun 15. Lancet. 2015. PMID: 26088268 Clinical Trial.
-
First Clinical Experience with ONO-4232: A Randomized, Double-blind, Placebo-controlled Healthy Volunteer Study of a Novel Lusitropic Agent for Acutely Decompensated Heart Failure.Clin Ther. 2016 May;38(5):1109-21. doi: 10.1016/j.clinthera.2016.02.019. Epub 2016 Mar 19. Clin Ther. 2016. PMID: 27001444 Clinical Trial.
-
Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects.Antimicrob Agents Chemother. 2015 Feb;59(2):1246-51. doi: 10.1128/AAC.04223-14. Epub 2014 Dec 15. Antimicrob Agents Chemother. 2015. PMID: 25512407 Free PMC article. Clinical Trial.
-
Clevidipine: a review of its use in the management of acute hypertension.Am J Cardiovasc Drugs. 2009;9(2):117-34. doi: 10.2165/00129784-200909020-00006. Am J Cardiovasc Drugs. 2009. PMID: 19331440 Review.
-
Functional autoantibody diseases: Basics and treatment related to cardiomyopathies.Front Biosci (Landmark Ed). 2019 Jan 1;24(1):48-95. doi: 10.2741/4709. Front Biosci (Landmark Ed). 2019. PMID: 30468647 Review.
Cited by
-
Cardiology's best friend: Using naturally occurring disease in dogs to understand heart disease in humans.J Mol Cell Cardiol Plus. 2025 Jul 4;13:100474. doi: 10.1016/j.jmccpl.2025.100474. eCollection 2025 Sep. J Mol Cell Cardiol Plus. 2025. PMID: 40686503 Free PMC article. Review.
-
Lack of efficacy of mono-mode of action therapeutics in COVID-19 therapy - How the lack of predictive power of preclinical cell and animal studies leads developments astray.Chem Biol Drug Des. 2022 Jan;99(1):32-45. doi: 10.1111/cbdd.13954. Epub 2021 Oct 31. Chem Biol Drug Des. 2022. PMID: 34549885 Free PMC article.
-
Aptamers-Diagnostic and Therapeutic Solution in SARS-CoV-2.Int J Mol Sci. 2022 Jan 26;23(3):1412. doi: 10.3390/ijms23031412. Int J Mol Sci. 2022. PMID: 35163338 Free PMC article. Review.
-
After the Hurricane: Anti-COVID-19 Drugs Development, Molecular Mechanisms of Action and Future Perspectives.Int J Mol Sci. 2024 Jan 6;25(2):739. doi: 10.3390/ijms25020739. Int J Mol Sci. 2024. PMID: 38255813 Free PMC article. Review.
-
Aptamers Against COVID-19: An Untested Opportunity.Mini Rev Med Chem. 2022;22(13):1708-1715. doi: 10.2174/1389557522666220112094951. Mini Rev Med Chem. 2022. PMID: 35023454 Free PMC article.
References
-
- McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs033. - DOI - PubMed
-
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in collaboration with the International Society for Heart And Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

