Effect of Zephyr Endobronchial Valves on Dyspnea, Activity Levels, and Quality of Life at One Year. Results from a Randomized Clinical Trial
- PMID: 32223724
- PMCID: PMC7328183
- DOI: 10.1513/AnnalsATS.201909-666OC
Effect of Zephyr Endobronchial Valves on Dyspnea, Activity Levels, and Quality of Life at One Year. Results from a Randomized Clinical Trial
Abstract
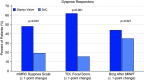
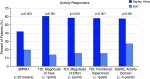
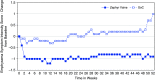
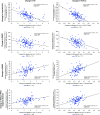
Rationale: Bronchoscopic lung volume reduction with Zephyr Valves improves lung function, exercise tolerance, and quality of life of patients with hyperinflated emphysema and little to no collateral ventilation.Objectives:Post hoc analysis of patient-reported outcomes (PROs), including multidimensional measures of dyspnea, activity, and quality of life, in the LIBERATE (Lung Function Improvement after Bronchoscopic Lung Volume Reduction with Pulmonx Endobronchial Valves used in Treatment of Emphysema) study are reported.Methods: A total of 190 patients with severe heterogeneous emphysema and little to no collateral ventilation in the target lobe were randomized 2:1 to the Zephyr Valve or standard of care. Changes in PROs at 12 months in the two groups were compared: dyspnea with the Transitional Dyspnea Index (TDI), focal score; the Chronic Obstructive Pulmonary Disease Assessment Test (CAT; breathlessness on hill/stairs); Borg; the EXAcerbations of Chronic pulmonary disease Tool-PRO, dyspnea domain; activity with the TDI, magnitude of task/effort/functional impairment, CAT (limited activities), and the St. George's Respiratory Questionnaire (SGRQ), activity domain; and psychosocial status with the SGRQ, impacts domain, and CAT (confidence and energy).Results: At 12 months, patients using the Zephyr Valve achieved statistically significant and clinically meaningful improvements in the SGRQ; CAT; and the TDI, focal score, compared with standard of care. Improvements in the SGRQ were driven by the impacts and activity domains (P < 0.05 and P < 0.001, respectively). Reduction in CAT was through improvements in breathlessness (P < 0.05), energy level (P < 0.05), activities (P < 0.001), and increased confidence when leaving home (P < 0.05). The TDI measures of effort, task, and functional impairment were uniformly improved (P < 0.001). The EXAcerbations of Chronic Pulmonary Disease Tool (EXACT)-PRO, dyspnea domain, was significantly improved in the Zephyr Valve group. Improvements correlated with changes in residual volume and residual volume/TLC ratio.Conclusions: Patients with severe hyperinflated emphysema achieving lung volume reductions with Zephyr Valves experience improvements in multidimensional scores for breathlessness, activity, and psychosocial parameters out to at least 12 months.Clinical trial registered with www.clinicaltrials.gov (NCT01796392).
Keywords: Zephyr Valve; chronic obstructive pulmonary disease; interventional bronchoscopy; patient-reported outcomes; severe emphysema.
Figures
References
-
- Dyspnea: mechanisms, assessment, and management. A consensus statement: American Thoracic Society. Am J Respir Crit Care Med. 1999;159:321–340. - PubMed
-
- Schönhofer B, Ardes P, Geibel M, Köhler D, Jones PW. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997;10:2814–2819. - PubMed
-
- Casaburi R. Impacting patient-centred outcomes in COPD: deconditioning. Eur Respir Rev. 2006;15:42–46.
-
- Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33(Suppl):S459–S471. [Discussion, pp. S493–S494] - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

