Children with reported penicillin allergy: Public health impact and safety of delabeling
- PMID: 32224207
- PMCID: PMC7255916
- DOI: 10.1016/j.anai.2020.03.012
Children with reported penicillin allergy: Public health impact and safety of delabeling
Abstract
Objective: To review the relevant literature related to children with reported penicillin allergy and highlight the different ways in which children could be delabeled and to evaluate the public health impact that a penicillin allergy has for children.
Data sources: Data for this review were obtained via PubMed searches and then retrieval of articles from their respective journals for further review.
Study selections: Studies regarding the safety of different ways to evaluate penicillin allergy in children were identified via PubMed searches. Any study that reported different ways of testing (3-tier, direct oral challenge, 5-day oral challenges) were included. This same format was used when selecting relevant articg:les related to the costs, prescription patterns, and stewardship trends associated with a penicillin allergy label.
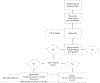
Results: This review found that penicillin allergy testing is a safe and effective way to delabel those with reported allergy. In children with low-risk allergy symptoms, a direct oral challenge approach may be optimal. In those children with a history of high-risk allergy symptoms, a 3-tiered approach is ideal. The review also found that there is a significant cost associated with reported penicillin allergy and that there are increased negative health benefits to those children with reported allergy.
Conclusion: Penicillin allergy is overdiagnosed, often incorrectly, and the label is frequently first applied during childhood. Targeting children for the removal of the incorrect penicillin allergy label provides a mechanism to reduce the use of broader-spectrum and less effective antibiotics.
Copyright © 2020 American College of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
Potential Conflicts of Interest: EJP is Drug Allergy Section Editor for uptodate.
Figures

References
-
- Kerr JR. Penicillin allergy: a study of incidence as reported by patients. Br J Clin Pract. 1994;48(1):5–7pmid:817998 - PubMed
-
- Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med. 2000;160(18):2819–2822pmid:11025792. - PubMed
-
- Mendelson LM. Adverse reactions to β-lactam antibiotics. Immunol Allergy Clin North Am, 18 (1998), pp. 745–757
-
- Vyles D, Chiu A, Simpson P, Nimmer M, Adams J, Brousseau DC. Parent Reported 131 Penicillin Allergy Symptoms in the Pediatric Emergency Department. Acad Pediatr 132 2017;17(3):251–5. 133 134 - PubMed
-
- Sogn DD. Results of the National Institute of Allergy and Infectious Diseases 142 Collaborative Clinical Trial to Test the Predictive Value of Skin Testing With Major and 143 Minor Penicillin Derivatives in Hospitalized Adults. Arch Intern Med Archives of 144 Internal Medicine 1992;152(5):1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

