Biomarkers for Duchenne muscular dystrophy: myonecrosis, inflammation and oxidative stress
- PMID: 32224496
- PMCID: PMC7063669
- DOI: 10.1242/dmm.043638
Biomarkers for Duchenne muscular dystrophy: myonecrosis, inflammation and oxidative stress
Abstract
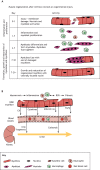
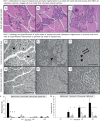
Duchenne muscular dystrophy (DMD) is a lethal, X-linked disease that causes severe loss of muscle mass and function in young children. Promising therapies for DMD are being developed, but the long lead times required when using clinical outcome measures are hindering progress. This progress would be facilitated by robust molecular biomarkers in biofluids, such as blood and urine, which could be used to monitor disease progression and severity, as well as to determine optimal drug dosing before a full clinical trial. Many candidate DMD biomarkers have been identified, but there have been few follow-up studies to validate them. This Review describes the promising biomarkers for dystrophic muscle that have been identified in muscle, mainly using animal models. We strongly focus on myonecrosis and the associated inflammation and oxidative stress in DMD muscle, as the lack of dystrophin causes repeated bouts of myonecrosis, which are the key events that initiate the resultant severe dystropathology. We discuss the early events of intrinsic myonecrosis, along with early regeneration in the context of histological and other measures that are used to quantify its incidence. Molecular biomarkers linked to the closely associated events of inflammation and oxidative damage are discussed, with a focus on research related to protein thiol oxidation and to neutrophils. We summarise data linked to myonecrosis in muscle, blood and urine of dystrophic animal species, and discuss the challenge of translating such biomarkers to the clinic for DMD patients, especially to enhance the success of clinical trials.
Keywords: Biomarkers; Blood; DMD; Dogs; Dystrophic mice; Inflammation; Muscle necrosis; Neutrophils; Oxidative stress; Rats; Urine.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

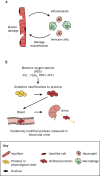
 , dismutation of which leads to the formation of hydrogen peroxide (H2O2) that is either catalysed by MPO to form the highly cytotoxic oxidant hypochlorous acid (HOCl), or is further oxidised to generate hydroxyl radicals (OH•). These oxidants can potentially exacerbate necrosis of dystrophic myofibres by the reversible and irreversible damage modifications that affect the function of cellular proteins. These modified proteins can enter circulation and are often excreted, therefore the measurement of these modifications in plasma and urine can be used as biomarkers of inflammation and oxidative stress in the muscle.
, dismutation of which leads to the formation of hydrogen peroxide (H2O2) that is either catalysed by MPO to form the highly cytotoxic oxidant hypochlorous acid (HOCl), or is further oxidised to generate hydroxyl radicals (OH•). These oxidants can potentially exacerbate necrosis of dystrophic myofibres by the reversible and irreversible damage modifications that affect the function of cellular proteins. These modified proteins can enter circulation and are often excreted, therefore the measurement of these modifications in plasma and urine can be used as biomarkers of inflammation and oxidative stress in the muscle.
References
-
- Aartsma-Rus A., Ferlini A., McNally E. M., Spitali P., Sweeney H. L. and Workshop participants (2018). 226(th) ENMC International Workshop: Towards validated and qualified biomarkers for therapy development for Duchenne muscular dystrophy 20-22 January 2017, Heemskerk, The Netherlands. Neuromuscul. Disord. 28, 77-86. 10.1016/j.nmd.2017.10.002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

