Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020
- PMID: 32224621
- PMCID: PMC7265766
- DOI: 10.1159/000507218
Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020
Abstract
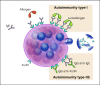
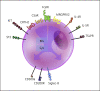
This update on chronic urticaria (CU) focuses on the prevalence and pathogenesis of chronic spontaneous urticaria (CSU), the expanding spectrum of patient-reported outcome measures (PROMs) for assessing CU disease activity, impact, and control, as well as future treatment options for CU. This update is needed, as several recently reported findings have led to significant advances in these areas. Some of these key discoveries were first presented at past meetings of the Collegium Internationale Allergologicum (CIA). New evidence shows that the prevalence of CSU is geographically heterogeneous, high in all age groups, and increasing. Several recent reports have helped to better characterize two endotypes of CSU: type I autoimmune (or autoallergic) CSU, driven by IgE to autoallergens, and type IIb autoimmune CSU, which is due to mast cell (MC)-targeted autoantibodies. The aim of treatment in CU is complete disease control with absence of signs and symptoms as well as normalization of quality of life (QoL). This is best monitored by the use of an expanding set of PROMs, to which the Angioedema Control Test, the Cholinergic Urticaria Quality of Life Questionnaire, and the Cholinergic Urticaria Activity Score have recently been added. Current treatment approaches for CU under development include drugs that inhibit the effects of signals that drive MC activation and accumulation, drugs that inhibit intracellular pathways of MC activation and degranulation, and drugs that silence MCs by binding to inhibitory receptors. The understanding, knowledge, and management of CU are rapidly increasing. The aim of this review is to provide physicians who treat CU patients with an update on where we stand and where we will go. Many questions and unmet needs remain to be addressed, such as the development of routine diagnostic tests for type I and type IIb autoimmune CSU, the global dissemination and consistent use of PROMs to assess disease activity, impact, and control, and the development of more effective and well-tolerated long-term treatments for all forms of CU.
Keywords: Angioedema; Patient-reported outcomes; Prevalence; Treatment; Wheals.
© 2020 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
M. Maurer has received honoraria (advisory board, speaker) and/or institutional grant/research support from Allakos, Amgen, Astra-Zeneca, Bayer, Dr. Pfleger, FAES, Genentech, GSK, Innate Pharma, Kyowa Kirin, Lilly, Merckle Recordati, Moxie, Novartis, Regeneron, Roche, Sanofi, MSD, UCB, and Uriach. M. Ferrer has received honoraria (advisory board, speaker) from Genentech, Menarini, Uriach, FAES, and MSD and has received a research grant and advisory and speaker fees from Novartis. K. Schäkel has received honoraria (advisory board, speaker) from ALK-Abelló, Almirall, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, and Novartis and grants/research support from Novartis. J. Gutermuth has received honoraria (advisory board, speaker) from Abbvie, Almirall, Celgene, Eli-Lilly, Janssen, MSD, Leo Pharma, Pierre-Fabre, Pfizer, Regeneron-Sanofi, and Thermo Fisher Scientific. K. Hartmann has received honoraria (advisory board, speaker) from ALK-Abelló, Allergopharma, Blueprint, Deciphera, Menarini, Novartis, and Takeda and institutional grant/research support from Euroimmun and Thermo Fisher Scientific. T. Jakob has received honoraria (advisory board, speaker) from ALK-Abelló, Allergopharma, Bencard/Allergy Therapeutics, Celgene, Novartis, and Thermo Fisher Scientific and grants/research support from ALK-Abelló, Bencard/Allergy Therapeutics, and Novartis. P. Kolkhir has received speaker fees from Novartis and Roche. D. Larenas-Linnemann has received honoraria (advisory board, speaker) and/or grants for guideline development support from Allakos, Alerquim, Astra-Zeneca, Boehringer Ingelheim, DBV, Diemsa, Glenmark, GSK, Menarini, Mylan, Novartis, Sanofi, and UCB. M. Sánchez-Borges has received honoraria from Allakos for advisory boards and speaker fees from Novartis. K. Weller has received honoraria (advisory board, speaker) from Dr. R. Pfleger, Essex Pharma (now MSD), Novartis, UCB, Uriach, and Moxie. T. Zuberbier has received honoraria (advisory board, speaker) from AstraZeneca, AbbVie, ALK-Abelló, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, FAES, HAL, Henkel, Kryolan, Leti, Lofarma, L'Oreal, Meda, Menarini, Merck, MSD, Novartis, Pfizer, Sanofi, Sanoflore, Stallergenes, Takeda, Teva, and UCB. M. Metz has received honoraria (advisory board, speaker) from Amgen, Aralez, argenx, Bayer, Beiersdorf, Celgene, Menlo, Moxie, Novartis, Roche, Sanofi, Shire, Siennabio, and Uriach. K. Eyerich, S. Eyerich, A. Kapp, H.-S. Park, G. Pejler, D. Simon, and H.-U. Simon have no conflicts of interest related to this paper.
Figures

References
-
- Fricke J, Avila G, Keller T, Weller K, Lau S, Maurer M, et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. 2019 Feb;75((2)):423–32. - PubMed
-
- Gaig P, Olona M, Muñoz Lejarazu D, Caballero MT, Domínguez FJ, Echechipia S, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14((3)):214–20. - PubMed
-
- Lapi F, Cassano N, Pegoraro V, Cataldo N, Heiman F, Cricelli I, et al. Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol. 2016 May;174((5)):996–1004. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

