Analysis of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas and Paired Normal Mucosae Reveals Cyclin D1 Deregulation and Compensatory Effect of Cyclin D2
- PMID: 32224897
- PMCID: PMC7226528
- DOI: 10.3390/cancers12040792
Analysis of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas and Paired Normal Mucosae Reveals Cyclin D1 Deregulation and Compensatory Effect of Cyclin D2
Abstract
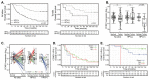
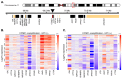
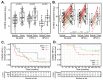
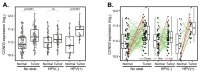
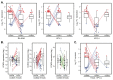
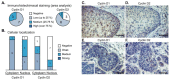
Aberrant regulation of the cell cycle is a typical feature of all forms of cancer. In head and neck squamous cell carcinoma (HNSCC), it is often associated with the overexpression of cyclin D1 (CCND1). However, it remains unclear how CCND1 expression changes between tumor and normal tissues and whether human papillomavirus (HPV) affects differential CCND1 expression. Here, we evaluated the expression of D-type cyclins in a cohort of 94 HNSCC patients of which 82 were subjected to whole genome expression profiling of primary tumors and paired normal mucosa. Comparative analysis of paired samples showed that CCND1 was upregulated in 18% of HNSCC tumors. Counterintuitively, CCND1 was downregulated in 23% of carcinomas, more frequently in HPV-positive samples. There was no correlation between the change in D-type cyclin expression and patient survival. Intriguingly, among the tumors with downregulated CCND1, one-third showed an increase in cyclin D2 (CCND2) expression. On the other hand, one-third of tumors with upregulated CCND1 showed a decrease in CCND2. Collectively, we have shown that CCND1 was frequently downregulated in HNSCC tumors. Furthermore, regardless of the HPV status, our data suggested that a change in CCND1 expression was alleviated by a compensatory change in CCND2 expression.
Keywords: 11q13 amplification; CCND1; CCND2; CCND3; D-type cyclins; cell cycle; head and neck squamous cell carcinoma; human papillomavirus; paired tumor-normal samples; patient survival.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures
References
-
- Hashibe M., Brennan P., Chuang S.C., Boccia S., Castellsague X., Chen C., Curado M.P., Dal Maso L., Daudt A.W., Fabianova E., et al. Interaction between Tobacco and Alcohol Use and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009;18:541–550. - PMC - PubMed
Grants and funding
- 16-29032A/Agentura Pro Zdravotnický Výzkum České Republiky/International
- 18-11908S/Grantová Agentura České Republiky/International
- Operational Programme Research, Development and Education under the project "Center for Tumor Ecology - Research of the Cancer Microenvironment Supporting Cancer Growth and Spread" (reg. No. CZ.02.1.01/0.0/0.0/16_019/0000785)/Ministerstvo Školství, Mládeže a Tělovýchovy/International
- Research and Development for Innovations Operational Program under project no. CZ.1.05/2.1.00/19.0400/Ministerstvo Školství, Mládeže a Tělovýchovy/International
LinkOut - more resources
Full Text Sources
Research Materials

