Tetraspanin CD9 is Regulated by miR-518f-5p and Functions in Breast Cell Migration and In Vivo Tumor Growth
- PMID: 32224917
- PMCID: PMC7226392
- DOI: 10.3390/cancers12040795
Tetraspanin CD9 is Regulated by miR-518f-5p and Functions in Breast Cell Migration and In Vivo Tumor Growth
Abstract
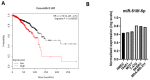
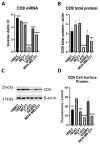
Breast cancer is the most commonly diagnosed and the second leading cause of cancer-related mortality among women worldwide. miR-518f-5p has been shown to modulate the expression of the metastasis suppressor CD9 in prostate cancer. However, the role of miR-518f-5p and CD9 in breast cancer is unknown. Therefore, this study aimed to elucidate the role of miR-518f-5p and the mechanisms responsible for decreased CD9 expression in breast cancer, as well as the role of CD9 in de novo tumor formation and metastasis. miR-518f-5p function was assessed using migration, adhesion, and proliferation assays. miR-518f-5p was overexpressed in breast cancer cell lines that displayed significantly lower CD9 expression as well as less endogenous CD9 3'UTR activity, as assessed using qPCR and dual luciferase assays. Transfection of miR-518f-5p significantly decreased CD9 protein expression and increased breast cell migration in vitro. Cd9 deletion in the MMTV/PyMT mouse model impaired tumor growth, but had no effect on tumor initiation or metastasis. Therefore, inhibition of miR-518f-5p may restore CD9 expression and aid in the treatment of breast cancer metastasis.
Keywords: CD9; breast cancer; miR-518f-5p; migration; regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Miyake M., Nakano K., Itoi S.I., Koh T., Taki T. Motility-related protein-1 (MRP-1/CD9) reduction as a factor of poor prognosis in breast cancer. Cancer Res. 1996;56:1244–1249. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

