Estrogen Receptors and Ubiquitin Proteasome System: Mutual Regulation
- PMID: 32224970
- PMCID: PMC7226411
- DOI: 10.3390/biom10040500
Estrogen Receptors and Ubiquitin Proteasome System: Mutual Regulation
Abstract
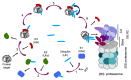
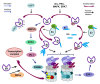
This review provides information on the structure of estrogen receptors (ERs), their localization and functions in mammalian cells. Additionally, the structure of proteasomes and mechanisms of protein ubiquitination and cleavage are described. According to the modern concept, the ubiquitin proteasome system (UPS) is involved in the regulation of the activity of ERs in several ways. First, UPS performs the ubiquitination of ERs with a change in their functional activity. Second, UPS degrades ERs and their transcriptional regulators. Third, UPS affects the expression of ER genes. In addition, the opportunity of the regulation of proteasome functioning by ERs-in particular, the expression of immune proteasomes-is discussed. Understanding the complex mechanisms underlying the regulation of ERs and proteasomes has great prospects for the development of new therapeutic agents that can make a significant contribution to the treatment of diseases associated with the impaired function of these biomolecules.
Keywords: cancer; immune proteasomes; membrane estrogen receptors; nuclear estrogen receptors; ubiquitin proteasome system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Szmyd M., Lloyd V., Hallman K., Aleck K., Mladenovik V., McKee C., Morse M., Bedgood T., Dinda S. The effects of black cohosh on the regulation of estrogen receptor (ERα) and progesterone receptor (PR) in breast cancer cells. Breast Cancer. 2018;10:1–11. doi: 10.2147/BCTT.S144865. - DOI - PMC - PubMed
-
- Jover-Mengual T., Castelló-Ruiz M., Burguete M.C., Jorques M., López-Morales M.A., Aliena-Valero A., Jurado-Rodríguez A., Pérez S., Centeno J.M., Miranda F.J., et al. Molecular mechanisms mediating the neuroprotective role of the selective estrogen receptor modulator, bazedoxifene, in acute ischemic stroke: A comparative study with 17β-estradiol. J. Steroid. Biochem. Mol. Biol. 2017;171:296–304. doi: 10.1016/j.jsbmb.2017.05.001. - DOI - PubMed
-
- Andersson A., Törnqvist A.E., Moverare-Skrtic S., Bernardi A.I., Farman H.H., Chambon P., Engdahl C., Lagerquist M.K., Windahl S.H., Carlsten H., et al. Roles of activating functions 1 and 2 of estrogen receptor α in lymphopoiesis. J. Endocrinol. 2018;236:99–109. doi: 10.1530/JOE-17-0372. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Government program of basic research in Cancer Research Institute of Tomsk National Research Medical Center of the Russian Academy of Sciences in 2020/Ministry of Science and Higher Education of Russian Federation/International
- Government program of basic research in Koltzov Institute of Developmental Biology of the Russian Academy of Sciences in 2020/Ministry of Science and Higher Education of Russian Federation/International
LinkOut - more resources
Full Text Sources

