Combination immunotherapy with anti-PD-L1 antibody and depletion of regulatory T cells during acute viral infections results in improved virus control but lethal immunopathology
- PMID: 32226027
- PMCID: PMC7105110
- DOI: 10.1371/journal.ppat.1008340
Combination immunotherapy with anti-PD-L1 antibody and depletion of regulatory T cells during acute viral infections results in improved virus control but lethal immunopathology
Abstract
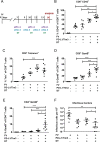
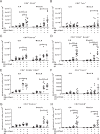
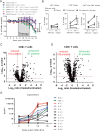
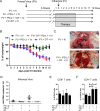
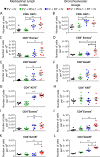
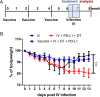
Combination immunotherapy (CIT) is currently applied as a treatment for different cancers and is proposed as a cure strategy for chronic viral infections. Whether such therapies are efficient during an acute infection remains elusive. To address this, inhibitory receptors were blocked and regulatory T cells depleted in acutely Friend retrovirus-infected mice. CIT resulted in a dramatic expansion of cytotoxic CD4+ and CD8+ T cells and a subsequent reduction in viral loads. Despite limited viral replication, mice developed fatal immunopathology after CIT. The pathology was most severe in the gastrointestinal tract and was mediated by granzyme B producing CD4+ and CD8+ T cells. A similar post-CIT pathology during acute Influenza virus infection of mice was observed, which could be prevented by vaccination. Melanoma patients who developed immune-related adverse events under immune checkpoint CIT also presented with expanded granzyme-expressing CD4+ and CD8+ T cell populations. Our data suggest that acute infections may induce immunopathology in patients treated with CIT, and that effective measures for infection prevention should be applied.
Conflict of interest statement
NO authors have competing interests.
Figures


References
-
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(19):12293–7. 10.1073/pnas.192461099 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

