Vanadium compounds in medicine
- PMID: 32226091
- PMCID: PMC7094629
- DOI: 10.1016/j.ccr.2014.12.002
Vanadium compounds in medicine
Abstract
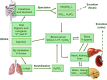
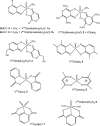
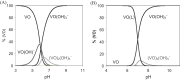
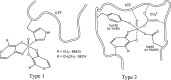
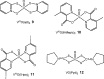
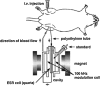
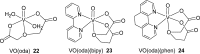
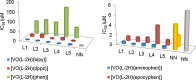
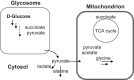
Vanadium is a transition metal that, being ubiquitously distributed in soil, crude oil, water and air, also found roles in biological systems and is an essential element in most living beings. There are also several groups of organisms which accumulate vanadium, employing it in their biological processes. Vanadium being a biological relevant element, it is not surprising that many vanadium based therapeutic drugs have been proposed for the treatment of several types of diseases. Namely, vanadium compounds, in particular organic derivatives, have been proposed for the treatment of diabetes, of cancer and of diseases caused by parasites. In this work we review the medicinal applications proposed for vanadium compounds with particular emphasis on the more recent publications. In cells, partly due to the similarity of vanadate and phosphate, vanadium compounds activate numerous signaling pathways and transcription factors; this by itself potentiates application of vanadium-based therapeutics. Nevertheless, this non-specific bio-activity may also introduce several deleterious side effects as in addition, due to Fenton's type reactions or of the reaction with atmospheric O2, VCs may also generate reactive oxygen species, thereby introducing oxidative stress with consequences presently not well evaluated, particularly for long-term administration of vanadium to humans. Notwithstanding, the potential of vanadium compounds to treat type 2 diabetes is still an open question and therapies using vanadium compounds for e.g. antitumor and anti-parasitic related diseases remain promising.
Keywords: Antiparasitic activity; Biological properties; Cancer therapy; Insulin-enhancing agents; Vanadium.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
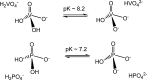




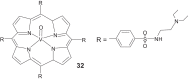
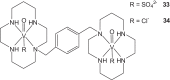

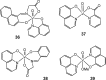
References
-
- Kiss T., Jakusch T., Hollender D., Dornyei A., Enyedy E.A., Costa Pessoa J., Sakurai H., Sanz-Medel A. Coord. Chem. Rev. 2008;252:1153.
-
- Rehder D. John Wiley & Sons; New York: 2008. Bioinorganic Vanadium Chemistry.
-
- Sakurai H., Yoshikawa Y., Yasui H. Chem. Soc. Rev. 2008;37:2383. - PubMed
-
- Rehder D. Dalton Trans. 2013;42:11749. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
