Vanadium: History, chemistry, interactions with α-amino acids and potential therapeutic applications
- PMID: 32226092
- PMCID: PMC7094547
- DOI: 10.1016/j.ccr.2018.06.002
Vanadium: History, chemistry, interactions with α-amino acids and potential therapeutic applications
Abstract
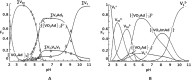
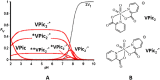
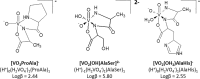
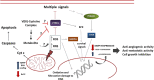
In the last 30 years, since the discovery that vanadium is a cofactor found in certain enzymes of tunicates and possibly in mammals, different vanadium-based drugs have been developed targeting to treat different pathologies. So far, the in vitro studies of the insulin mimetic, antitumor and antiparasitic activity of certain compounds of vanadium have resulted in a great boom of its inorganic and bioinorganic chemistry. Chemical speciation studies of vanadium with amino acids under controlled conditions or, even in blood plasma, are essential for the understanding of the biotransformation of e.g. vanadium antidiabetic complexes at the physiological level, providing clues of their mechanism of action. The present article carries out a bibliographical research emphaticizing the chemical speciation of the vanadium with different amino acids and reviewing also some other important aspects such as its chemistry and therapeutical applications of several vanadium complexes.
Keywords: 2,2′-bipy, 2,2-bipyridine; 6-mepic, 6-methylpicolinic acid; Ad, adenosine; Ala, alanine; Ala-Gly, alanylglycine; Ala-His, alanylhistidine; Ala-Ser, alanylserine; Amino acids; Antidiabetics; Antitumors; Asp, aspartic acid; BEOV, bis(ethylmaltolate)oxovanadium(IV); Chemical speciation; Cys, cysteine; Cyt, citrate; DMF, N,N-dimethylformamide; DNA, deoxyribonucleic acid; EPR, Electron Paramagnetic Resonance; G, Gauss; Glu, glutamic acid; Gly, glycine; GlyAla, glycylalanine; GlyGly, glycylglycine; GlyGlyCys, glycylglycylcysteine; GlyGlyGly, glycylglycylglycine; GlyGlyHis, glycylglycylhistidine; GlyPhe, glycylphenylalanine; GlyTyr, glycyltyrosine; GlyVal, glycylvaline; HIV, human immunodeficiency virus; HSA, albumin; Hb, hemoglobin; His, histidine; HisGlyGly, histidylglycylglycine; Ig, immunoglobulins; Im, imidazole; L-Glu(γ)HXM, l-glutamic acid γ-monohydroxamate; LD50, the amount of a toxic agent (such as a poison, virus, or radiation) that is sufficient to kill 50 percent of population of animals; Lac, lactate; MeCN, acetonitrile; NADH and NAD+, nicotinamide adenine dinucleotide; NEP, neutral endopeptidas; NMR, Nuclear Magnetic Resonance; Ox, oxalate; PI3K, phosphoinositide 3-kinase; PTP1B, protein tyrosine phosphatase 1B; Pic, picolinic acid; Pro, proline; Pro-Ala, prolylalanine; RNA, ribonucleic acid; SARS, severe acute respiratory syndrome; Sal-Ala, N-salicylidene-l-alaninate; SalGly, salicylglycine; SalGlyAla, salicylglycylalanine; Ser, serine; T, Tesla; THF, tetrahydrofuran; Thr, threonine; VBPO, vanadium bromoperoxidases; VanSer, Schiff base formed from o-vanillin and l-serine; Vanadium complexes; acac, acetylacetone; dhp, 1,2-dimethyl-3-hydroxy-4(1H)-pyridinone; dipic, dipicolinic acid; dmpp, 1,2-dimethyl-3-hydroxy-4-pyridinonate; hTf, transferring; hpno, 2-hydroxypyridine-N-oxide; l.m.m., low molecular mass; mal, maltol; py, pyridine; sal-l-Phe, N-salicylidene-l-tryptophanate; salGlyGly, N-salicylideneglycylglycinate; salSer, N-salicylideneserinate; salTrp, N-salicylidene-L tryptophanate; salVal, N-salicylidene-l-valinate; salophen, N,N′-bis(salicylidene)-o-phenylenediamine; saltrp, N-salicylidene-l-tryptophanate; γ-PGA, poly-γ-glutamic acid.
© 2018 Elsevier B.V. All rights reserved.
Figures















References
-
- F. Cotton, G. Wilkinson. Química Inorgánica Avanzada, Capítulo 21. 4ta Edición, Limusa Editorial, 1999, pp. 855–869.
-
- Imtiaz M., Rizwan M.S., Xiong S., Li H., Ashraf M., Shahzad S.M., Shahzad M., Rizwan M., Tu S. Vanadium, recent advancements and research prospects: a review. Environ. Int. 2015;80:79–88. - PubMed
-
- E.F. Baroch. Vanadium and Vanadium Alloys. Kirk-Othmer. Encyclopedia of Chemical Technology, vol. 0. Wiley Editorial, 2006, pp. 1–21.
-
- Rehder D. The role of vanadium in biology. Critical review. Metallomics. 2015;7:730–742. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
