Current status and future development of solvent-based carbon capture
- PMID: 32226642
- PMCID: PMC7089636
- DOI: 10.1007/s40789-017-0159-0
Current status and future development of solvent-based carbon capture
Abstract
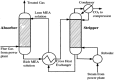
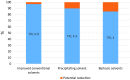
Solvent-based carbon capture is the most commercially-ready technology for economically and sustainably reaching carbon emission reduction targets in the power sector. Globally, the technology has been deployed to deal with flue gases from large scale power plants and different carbon-intensive industries. The success of the technology is due to significant R&D activities on the process development and decades of industrial experience on acid gas removal processes from gaseous mixtures. In this paper, current status of PCC based on chemical absorption-commercial deployment and demonstration projects, analysis of different solvents and process configurations-is reviewed. Although some successes have been recorded in developing this technology, its commercialization has been generally slow as evidenced in the cancellation of high profile projects across the world. This is partly due to the huge cost burden of the technology and unpredictable government policies. Different research directions, namely new process development involving process intensification, new solvent development and a combination of both, are discussed in this paper as possible pathways for reducing the huge cost of the technology.
Keywords: Chemical absorption; Post-combustion CO2 capture (PCC); Process intensification; Solvents.
© The Author(s) 2017.
Figures


References
-
- Aboudheir A, Tontiwachwuthikul P, Chakma A, Idem R. Kinetics of the reactive absorption of carbon dioxide in high CO2 -loaded, concentrated aqueous monoethanolamine solutions. Chem Eng Sci. 2003;58:5195–5210. doi: 10.1016/j.ces.2003.08.014. - DOI
-
- Agarwal L, Pavani V, Rao D, Kaistha N. Process intensification in HiGee absorption and distillation: design procedure and applications. Ind Eng Chem Res. 2010;49(100):46–58.
-
- Ahn H, Luberti M, Liu Z, Brandani S. Process configuration studies of the amine capture process for coal-fired power plants. Int J Greenh Gas Control. 2013;16:29–40. doi: 10.1016/j.ijggc.2013.03.002. - DOI
-
- Alstom (2009) We Energies’ Pleasant Prairie power plant: pilot project captures 90% of CO2. http://www.alstom.com/press-centre/2009/10/We-Energies-Pleasant-Prairie-.... Accessed Feb 2016
-
- Alstom (2011) Alstom announces successful results of Mountaineer Carbon Capture and Sequestration (CCS) Project. http://www.alstom.com/press-centre/2011/5/alstom-announces-sucessful-res.... Accessed Feb 2016
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
