Isradipine Versus Placebo in Early Parkinson Disease: A Randomized Trial
- PMID: 32227247
- PMCID: PMC7465126
- DOI: 10.7326/M19-2534
Isradipine Versus Placebo in Early Parkinson Disease: A Randomized Trial
Abstract
Background: Studies suggest that dihydropyridine calcium-channel blockers may be associated with reduced risk for Parkinson disease (PD).
Objective: To assess the effect of isradipine, a dihydropyridine calcium-channel blocker, on the rate of clinical progression of PD.
Design: Multicenter, randomized, parallel-group, double-blind, placebo-controlled trial. (ClinicalTrials.gov: NCT02168842).
Setting: 57 Parkinson Study Group sites in North America.
Participants: Patients with early-stage PD (duration <3 years) who were not taking dopaminergic medications at enrollment.
Intervention: 5 mg of immediate-release isradipine twice daily or placebo for 36 months.
Measurements: The primary outcome was change in the Unified Parkinson's Disease Rating Scale (UPDRS) parts I to III score measured in the antiparkinson medication "ON" state between baseline and 36 months. Secondary outcomes included time to initiation and use of antiparkinson medications, time to onset of motor complications, change in nonmotor disability, and quality-of-life measures.
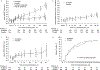
Results: 336 patients were randomly assigned (mean age, 62 years [SD, 9]; 68% men; disease duration, 0.9 year [SD, 0.7]; mean UPDRS part I to III score, 23.1 [SD, 8.6]); 95% of patients completed the study. Adjusted least-squares mean changes in total UPDRS score in the antiparkinson medication ON state over 36 months for isradipine and placebo recipients were 2.99 (95% CI, 0.95 to 5.03) points versus 3.26 (CI, 1.25 to 5.26) points, respectively, with a treatment effect of -0.27 (CI, -3.02 to 2.48) point (P = 0.85). Statistical adjustment for antiparkinson medication use did not change the findings. Secondary outcomes showed no effect of isradipine treatment. The most common adverse effects of isradipine were edema and dizziness.
Limitation: The isradipine dose may have been insufficient to engage the target calcium channels associated with neuroprotective effects.
Conclusion: Long-term treatment with immediate-release isradipine did not slow the clinical progression of early-stage PD.
Primary funding source: National Institute of Neurological Disorders and Stroke.
Figures

References
-
- de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. - PubMed
-
- Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–6. - PubMed
-
- Dodel RC, Singer M, Köhne-Volland R, et al. The economic impact of Parkinson’s disease: an estimation based on a 3-month prospective analysis. Pharmacoeconomics. 1998;14:299–312. - PubMed
-
- Huse DM, Schulman K, Orsini L, et al. Burden of illness in Parkinson’s disease. Mov Disord. 2005;20:1449–54. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
