Neurological manifestations of Erdheim-Chester Disease
- PMID: 32227455
- PMCID: PMC7187721
- DOI: 10.1002/acn3.51014
Neurological manifestations of Erdheim-Chester Disease
Abstract
Objective: To characterize the spectrum of neurologic involvement in Erdheim-Chester Disease (ECD), a treatable inflammatory neoplasm of histiocytes.
Methods: Sixty-two patients with ECD were prospectively enrolled in a natural history study that facilitated collection of clinical, imaging, laboratory, neurophysiologic, and pathologic data.
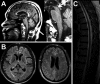
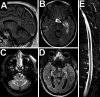
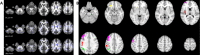
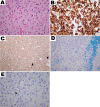
Results: Ninety-four percent of the patients had neurologic abnormalities on examination or imaging, and 22% had neurologic symptoms as the initial presentation of ECD. The most common neurologic findings were cognitive impairment, peripheral neuropathy, pyramidal tract signs, cranial nerve involvement, and cerebellar ataxia. Imaging revealed atrophy and demyelination along with focal lesions that were located throughout the nervous system, dura, and extra-axial structures. The BRAF V600E variant correlated with cerebral atrophy. Brain pathology revealed lipid-laden, phagocytic macrophages (histiocytes) accompanied by demyelination and axonal degeneration.
Interpretation: In patients with ECD, neurologic morbidity is common and contributes significantly to disability. Since neurologic symptoms can be the presenting feature of ECD and, given the mean delay in ECD diagnosis is 4.2 years, it is critical that neurologists consider of ECD and other histiocytosis in patients with inflammatory, infectious, or neoplastic-appearing white matter. Furthermore, given the broad spectrum of neurologic involvement, neurologists have an important role in a team of specialists treating ECD patients.
Published 2020. This article is a U.S Government work and is in the public domain in the USA. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
All authors report no conflicts of interest relevant to this work.
Figures
References
-
- Chester W. über Lipoidgranulomatose. Virchows Arch Path Anat 1930;279:561–602.
-
- Haroche J, Cohen‐Aubart F, Charlotte F, et al. The histiocytosis Erdheim‐Chester disease is an inflammatory myeloid neoplasm. Expert Rev Clin Immunol 2015;11:1033–1042. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
