Catalytic Hydroetherification of Unactivated Alkenes Enabled by Proton-Coupled Electron Transfer
- PMID: 32227658
- PMCID: PMC7451027
- DOI: 10.1002/anie.202003959
Catalytic Hydroetherification of Unactivated Alkenes Enabled by Proton-Coupled Electron Transfer
Abstract
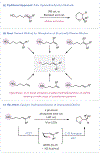
We report a catalytic, light-driven method for the intramolecular hydroetherification of unactivated alkenols to furnish cyclic ether products. These reactions occur under visible-light irradiation in the presence of an IrIII -based photoredox catalyst, a Brønsted base catalyst, and a hydrogen-atom transfer (HAT) co-catalyst. Reactive alkoxy radicals are proposed as key intermediates, generated by direct homolytic activation of alcohol O-H bonds through a proton-coupled electron-transfer mechanism. This method exhibits a broad substrate scope and high functional-group tolerance, and it accommodates a diverse range of alkene substitution patterns. Results demonstrating the extension of this catalytic system to carboetherification reactions are also presented.
Keywords: alcohols; ethers; hydroetherification; photocatalysis; radicals.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Elliott MC, J. Chem. Soc., Perkin Trans 1 1998, 4175–4200;
- Lorente A, Lamariano-Merketegi J, Albericio F, Álvarez M, Chem. Rev 2013, 113, 4567–4610. - PubMed
-
- Hintermann L, Top. Organomet. Chem 2010, 31, 123–155;
- Beller M, Seayad J, Tillack A, Jiao H, Angew. Chem. Int. Ed 2004, 43, 3368–3398; - PubMed
- Angew. Chem 2004, 116, 3448–3479;
- Zeng X, Chem. Rev 2013, 113, 6864–6900; - PubMed
- Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF, Org. Lett 2006, 8, 4179–4182. - PubMed
-
- Qian H, Han X, Widenhoefer RA, J. Am. Chem. Soc 2004, 126, 9536–9537; - PubMed
- Sevov CS, Hartwig JF, J. Am. Chem. Soc 2013, 135, 9303–9306; - PubMed
- Schlüter J, Blazejak M, Boeck F, Hintermann L, Angew. Chem. Int. Ed 2015, 54, 4014–4017; - PubMed
- Angew. Chem 2015, 127, 4086–4089;
- Marcyk PT, Cook SP, Org. Lett 2019, 21, 1547–1550. - PMC - PubMed
-
- Leger PR, Murphy RA, Pushkarskaya E, Sarpong R, Chem. Eur. J 2015, 21, 4377–4383; - PubMed
- Fujita S, Abe M, Shibuya M, Yamamoto Y, Org. Lett 2015, 17, 3822–3825; - PubMed
- Shigehisa H, Hayashi M, Ohkawa H, Suzuki T, Okayasu H, Mukai M, Yamazaki A, Kawai R, Kikuchi H, Satoh Y, Fukuyama A, Hiroya K, J. Am. Chem. Soc 2016, 138, 10597–10604; - PubMed
- Luo C, Bandar JS, J. Am. Chem. Soc 2018, 140, 3547–3550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources