From Pd(OAc)2 to Chiral Catalysts: The Discovery and Development of Bifunctional Mono-N-Protected Amino Acid Ligands for Diverse C-H Functionalization Reactions
- PMID: 32227915
- PMCID: PMC7738004
- DOI: 10.1021/acs.accounts.9b00621
From Pd(OAc)2 to Chiral Catalysts: The Discovery and Development of Bifunctional Mono-N-Protected Amino Acid Ligands for Diverse C-H Functionalization Reactions
Abstract
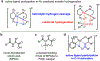
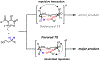
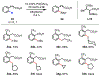
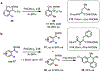
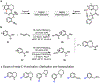
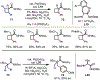
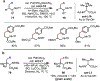
The functionalization of unactivated carbon-hydrogen bonds is a transformative strategy for the rapid construction of molecular complexity given the ubiquitous presence of C-H bonds in organic molecules. It represents a powerful tool for accelerating the synthesis of natural products and bioactive compounds while reducing the environmental and economic costs of synthesis. At the same time, the ubiquity and strength of C-H bonds also present major challenges toward the realization of transformations that are both highly selective and efficient. The development of practical C-H functionalization reactions has thus remained a compelling yet elusive goal in organic chemistry for over a century.Specifically, the capability to form useful new C-C, C-N, C-O, and C-X bonds via direct C-H functionalization would have wide-ranging impacts in organic synthesis. Palladium is especially attractive as a catalyst for such C-H functionalizations because of the diverse reactivity of intermediate palladium-carbon bonds. Early efforts using cyclopalladation with Pd(OAc)2 and related salts led to the development of many Pd-catalyzed C-H functionalization reactions. However, Pd(OAc)2 and other simple Pd salts perform only racemic transformations, which prompted a long search for effective chiral catalysts dating back to the 1970s. Pd salts also have low reactivity with synthetically useful substrates. To address these issues, effective and reliable ligands capable of accelerating and improving the selectivity of Pd-catalyzed C-H functionalizations are needed.In this Account, we highlight the discovery and development of bifunctional mono-N-protected amino acid (MPAA) ligands, which make great strides toward addressing these two challenges. MPAAs enable numerous Pd(II)-catalyzed C(sp2)-H and C(sp3)-H functionalization reactions of synthetically relevant substrates under operationally practical conditions with excellent stereoselectivity when applicable. Mechanistic studies indicate that MPAAs operate as unique bifunctional ligands for C-H activation in which both the carboxylate and amide are coordinated to Pd. The N-acyl group plays an active role in the C-H cleavage step, greatly accelerating C-H activation. The rigid MPAA chelation also results in a predictable transfer of chiral information from a single chiral center on the ligand to the substrate and permits the development of a rational stereomodel to predict the stereochemical outcome of enantioselective reactions.We also describe the application of MPAA-enabled C-H functionalization in total synthesis and provide an outlook for future development in this area. We anticipate that MPAAs and related next-generation ligands will continue to stimulate development in the field of Pd-catalyzed C-H functionalization.
Figures


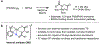



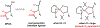
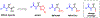
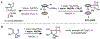
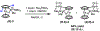
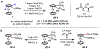

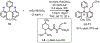
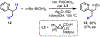






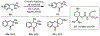

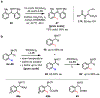
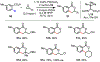
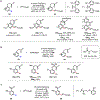





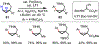
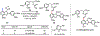


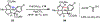
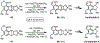
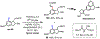
References
-
- Arndtsen BA; Bergman RG; Mobley TA; Peterson TH, Selective Intermolecular Carbon-Hydrogen Bond Activation by Synthetic Metal Complexes in Homogeneous Solution. Acc. Chem. Res 1995, 28, 154–162
- Davies HML; Manning JR, Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 2008, 451, 417–424 - PMC - PubMed
- Daugulis O; Do H-Q; Shabashov D, Palladium- and Copper-Catalyzed Arylation of Carbon–Hydrogen Bonds. Acc. Chem. Res 2009, 42, 1074–1086 - PMC - PubMed
- Roizen JL; Harvey ME; Du Bois J, Metal-Catalyzed Nitrogen-Atom Transfer Methods for the Oxidation of Aliphatic C–H Bonds. Acc. Chem. Res 2012, 45, 911–922 - PMC - PubMed
- Daugulis O; Roane J; Tran LD, Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon–Hydrogen Bonds. Acc. Chem. Res 2015, 48, 1053–1064 - PMC - PubMed
- Hartwig JF, Catalyst-Controlled Site-Selective Bond Activation. Acc. Chem. Res 2017, 50, 549–555 - PMC - PubMed
- Davies HML; Morton D, Collective Approach to Advancing C–H Functionalization. ACS Central Science 2017, 3, 936–943. - PMC - PubMed
-
- Chen X; Engle KM; Wang D-H; Yu J-Q, Palladium(II)-Catalyzed C–H Activation/C–C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed 2009, 48, 5094–5115 - PMC - PubMed
- Lyons TW; Sanford MS, Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions. Chem. Rev 2010, 110, 1147–1169 - PMC - PubMed
- He J; Wasa M; Chan KSL; Shao Q; Yu J-Q, Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chemical Reviews 2016. - PMC - PubMed
-
- Berrisford DJ; Bolm C; Sharpless KB, Ligand-Accelerated Catalysis. Angewandte Chemie International Edition 1995, 34, 1059–1070.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

