GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 Ab
- PMID: 32228854
- PMCID: PMC7108862
- DOI: 10.7554/eLife.49392
GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 Ab
Abstract
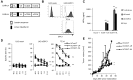
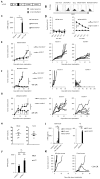
Current xenogeneic mouse models cannot evaluate on-target off-tumor adverse effect, hindering the development of chimeric antigen receptor (CAR) T cell therapies for solid tumors, due to limited human/mouse cross-reactivity of antibodies used in CAR and sever graft-versus-host disease induced by administered human T cells. We have evaluated safety and antitumor efficacy of CAR-T cells targeting glypican-1 (GPC1) overexpressed in various solid tumors. GPC1-specific human and murine CAR-T cells generated from our original anti-human/mouse GPC1 antibody showed strong antitumor effects in xenogeneic and syngeneic mouse models, respectively. Importantly, the murine CAR-T cells enhanced endogenous T cell responses against a non-GPC1 tumor antigen through the mechanism of antigen-spreading and showed synergistic antitumor effects with anti-PD-1 antibody without any adverse effects in syngeneic models. Our study shows the potential of GPC1 as a CAR-T cell target for solid tumors and the importance of syngeneic and xenogeneic models for evaluating their safety and efficacy.
Keywords: CAR-T cells; adverse effects; anti-PD-1 Ab; cancer biology; combination immunotherapy; human; mouse; solid tumor.
© 2020, Kato et al.
Conflict of interest statement
DK, TY, TI, YK, KM, KT, YT, MT, HK, HA, KT, SS, TN, RN, TN, YK No competing interests declared
Figures








References
-
- Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Kalos M, June CH. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunology Research. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. - DOI - PMC - PubMed
-
- Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D'Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B. Regression of glioblastoma after chimeric antigen receptor T-Cell therapy. New England Journal of Medicine. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. - DOI - PMC - PubMed
-
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, Carroll RG, Riley JL, Pastan I, June CH. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. PNAS. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. - DOI - PMC - PubMed
-
- Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, Feldman SA, Restifo NP, Rosenberg SA. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. Journal of Clinical Investigation. 2010;120:3953–3968. doi: 10.1172/JCI43490. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

