Approaching boiling point stability of an alcohol dehydrogenase through computationally-guided enzyme engineering
- PMID: 32228861
- PMCID: PMC7164962
- DOI: 10.7554/eLife.54639
Approaching boiling point stability of an alcohol dehydrogenase through computationally-guided enzyme engineering
Abstract
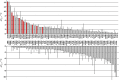
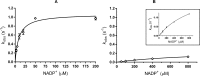
Enzyme instability is an important limitation for the investigation and application of enzymes. Therefore, methods to rapidly and effectively improve enzyme stability are highly appealing. In this study we applied a computational method (FRESCO) to guide the engineering of an alcohol dehydrogenase. Of the 177 selected mutations, 25 mutations brought about a significant increase in apparent melting temperature (ΔTm ≥ +3 °C). By combining mutations, a 10-fold mutant was generated with a Tm of 94 °C (+51 °C relative to wild type), almost reaching water's boiling point, and the highest increase with FRESCO to date. The 10-fold mutant's structure was elucidated, which enabled the identification of an activity-impairing mutation. After reverting this mutation, the enzyme showed no loss in activity compared to wild type, while displaying a Tm of 88 °C (+45 °C relative to wild type). This work demonstrates the value of enzyme stabilization through computational library design.
Keywords: E. coli; alcohol dehydrogenase; biocatalysis; biotechnology; cofactor; computational biology; enzyme engineering; molecular biophysics; oxidations; structural biology; systems biology.
© 2020, Aalbers et al.
Conflict of interest statement
FA, MF, SR, MT, JG, AV, AM, MF No competing interests declared, SB A patent application on the original ADH was filed by c-LEcta (WO 2019/012095)
Figures







References
-
- Arabnejad H, Lago MD, Jekel PA, Floor RJ, Thunnissen A, Terwisscha Van Scheltinga AC, Wijma HJ, Janssen DB. A robust cosolvent-compatible halohydrin dehalogenase by computational library design. Protein Engineering, Design & Selection : PEDS. 2017;30:175–189. doi: 10.1093/protein/gzw068. - DOI - PubMed
-
- Bednar D, Beerens K, Sebestova E, Bendl J, Khare S, Chaloupkova R, Prokop Z, Brezovsky J, Baker D, Damborsky J. FireProt: energy- and Evolution-Based computational design of thermostable Multiple-Point mutants. PLOS Computational Biology. 2015;11:e1004556. doi: 10.1371/journal.pcbi.1004556. - DOI - PMC - PubMed
-
- Bhatia C, Oerum S, Bray J, Kavanagh KL, Shafqat N, Yue W, Oppermann U. Towards a systematic analysis of human short-chain dehydrogenases/reductases (SDR): Ligand identification and structure-activity relationships. Chemico-Biological Interactions. 2015;234:114–125. doi: 10.1016/j.cbi.2014.12.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

