Accelerated partner therapy (APT) partner notification for people with Chlamydia trachomatis: protocol for the Limiting Undetected Sexually Transmitted infections to RedUce Morbidity (LUSTRUM) APT cross-over cluster randomised controlled trial
- PMID: 32229523
- PMCID: PMC7170609
- DOI: 10.1136/bmjopen-2019-034806
Accelerated partner therapy (APT) partner notification for people with Chlamydia trachomatis: protocol for the Limiting Undetected Sexually Transmitted infections to RedUce Morbidity (LUSTRUM) APT cross-over cluster randomised controlled trial
Abstract
Introduction: Partner notification (PN) is a process aiming to identify, test and treat the sex partners of people (index patients) with sexually transmitted infections (STIs). Accelerated partner therapy (APT) is a PN method whereby healthcare professionals assess sex partners, by telephone consultation, before giving the index patient antibiotics and STI self-sampling kits to deliver to their sex partner(s). The Limiting Undetected Sexually Transmitted infections to RedUce Morbidity programme aims to determine the effectiveness of APT in heterosexual women and men with chlamydia and determine whether APT could affect Chlamydia trachomatis transmission at population level.
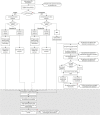
Methods and analysis: This protocol describes a cross-over cluster randomised controlled trial of APT, offered as an additional PN method, compared with standard PN. The trial is accompanied by an economic evaluation, transmission dynamic modelling and a qualitative process evaluation involving patients, partners and healthcare professionals. Clusters are 17 sexual health clinics in areas of England and Scotland with contrasting patient demographics. We will recruit 5440 heterosexual women and men with chlamydia, aged ≥16 years.The primary outcome is the proportion of index patients testing positive for C. trachomatis 12-16 weeks after the PN consultation. Secondary outcomes include: proportion of sex partners treated; cost effectiveness; model-predicted chlamydia prevalence; experiences of APT.The primary outcome analysis will be by intention-to-treat, fitting random effects logistic regression models that account for clustering of index patients within clinics and trial periods. The transmission dynamic model will be used to predict change in chlamydia prevalence following APT. The economic evaluation will use mathematical modelling outputs, taking a health service perspective. Qualitative data will be analysed using interpretative phenomenological analysis and framework analysis.
Ethics and dissemination: This protocol received ethical approval from London-Chelsea Research Ethics Committee (18/LO/0773). Findings will be published with open access licences.
Trial registration number: ISRCTN15996256.
Keywords: RCT; STIs; accelerated partner therapy; chlamydia; partner notification; transmission.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- UNAIDS Sexually transmitted diseases: policies and principles for prevention and care. Geneva, 1999. Available: https://www.unaids.org/en/resources/documents/1999/19990519_una97-6_en.pdf
-
- Althaus CL, Turner KME, Mercer CH, et al. . Effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable sexually transmitted infections: observational study, systematic reviews and mathematical modelling. Health Technol Assess 2014;18:1–100. vii–viii 10.3310/hta18020 - DOI - PMC - PubMed
-
- Public Health England Table 1: STI diagnoses and rates in England by gender, 2009 to 2018. London, 2019. Available: https://www.gov.uk/government/statistics/sexually-transmitted-infections...
-
- Health Protection Scotland Surveillance report genital Chlamydia and gonorrhoea infection in Scotland: laboratory diagnoses 2009-2018, 2019. Available: https://www.hps.scot.nhs.uk/web-resources-container/genital-chlamydia-an...
-
- Public Health Wales HIV and STI trends in Wales - Surveillance Report [Internet], 2018. Available: http://www.wales.nhs.uk/sitesplus/documents/888/HIVandSTItrendsinWalesRe...
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
