A Legionella effector kinase is activated by host inositol hexakisphosphate
- PMID: 32229585
- PMCID: PMC7196655
- DOI: 10.1074/jbc.RA120.013067
A Legionella effector kinase is activated by host inositol hexakisphosphate
Abstract
The transfer of a phosphate from ATP to a protein substrate, a modification known as protein phosphorylation, is catalyzed by protein kinases. Protein kinases play a crucial role in virtually every cellular activity. Recent studies of atypical protein kinases have highlighted the structural similarity of the kinase superfamily despite notable differences in primary amino acid sequence. Here, using a bioinformatics screen, we searched for putative protein kinases in the intracellular bacterial pathogen Legionella pneumophila and identified the type 4 secretion system effector Lpg2603 as a remote member of the protein kinase superfamily. Employing an array of biochemical and structural biology approaches, including in vitro kinase assays and isothermal titration calorimetry, we show that Lpg2603 is an active protein kinase with several atypical structural features. Importantly, we found that the eukaryote-specific host signaling molecule inositol hexakisphosphate (IP6) is required for Lpg2603 kinase activity. Crystal structures of Lpg2603 in the apo-form and when bound to IP6 revealed an active-site rearrangement that allows for ATP binding and catalysis. Our results on the structure and activity of Lpg2603 reveal a unique mode of regulation of a protein kinase, provide the first example of a bacterial kinase that requires IP6 for its activation, and may aid future work on the function of this effector during Legionella pathogenesis.
Keywords: Legionella; allosteric regulation; bacterial pathogenesis; bacterial protein kinase; effector; enzyme structure; inositol hexikisphosphate; inositol phosphate.
© 2020 Sreelatha et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

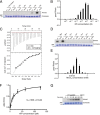
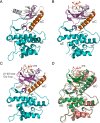
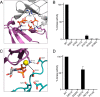
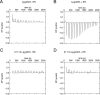

References
-
- Tagliabracci V. S., Wiley S. E., Guo X., Kinch L. N., Durrant E., Wen J., Xiao J., Cui J., Nguyen K. B., Engel J. L., Coon J. J., Grishin N., Pinna L. A., Pagliarini D. J., and Dixon J. E. (2015) A single kinase generates the majority of the secreted phosphoproteome. Cell 161, 1619–1632 10.1016/j.cell.2015.05.028 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

