Chronic Lymphocytic Leukemia
- PMID: 32229611
- PMCID: PMC7849345
- DOI: 10.1101/cshperspect.a035220
Chronic Lymphocytic Leukemia
Abstract
Patients with chronic lymphocytic leukemia can be divided into three categories: those who are minimally affected by the problem, often never requiring therapy; those that initially follow an indolent course but subsequently progress and require therapy; and those that from the point of diagnosis exhibit an aggressive disease necessitating treatment. Likewise, such patients pass through three phases: development of the disease, diagnosis, and need for therapy. Finally, the leukemic clones of all patients appear to require continuous input from the exterior, most often through membrane receptors, to allow them to survive and grow. This review is presented according to the temporal course that the disease follows, focusing on those external influences from the tissue microenvironment (TME) that support the time lines as well as those internal influences that are inherited or develop as genetic and epigenetic changes occurring over the time line. Regarding the former, special emphasis is placed on the input provided via the B-cell receptor for antigen and the C-X-C-motif chemokine receptor-4 and the therapeutic agents that block these inputs. Regarding the latter, prominence is laid upon inherited susceptibility genes and the genetic and epigenetic abnormalities that lead to the developmental and progression of the disease.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
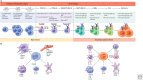
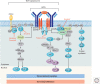
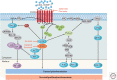

References
-
- Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ, Davis Z, van Gastel-Mol EJ, Tresoldi C, Chu CC, et al. 2012. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: A molecular classification with implications for targeted therapies. Blood 119: 4467–4475. 10.1182/blood-2011-11-393694 - DOI - PMC - PubMed
-
- Agathangelidis A, Ljungström V, Scarfò L, Fazi C, Gounari M, Pandzic T, Sutton L-A, Stamatopoulos K, Tonon G, Rosenquist R, et al. 2018. Highly similar genomic landscapes in monoclonal B-cell lymphocytosis and ultra-stable chronic lymphocytic leukemia with low frequency of driver mutations. Haematologica 103: 865–873. 10.3324/haematol.2017.177212 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
