Next-Generation Influenza Vaccines
- PMID: 32229612
- PMCID: PMC8327825
- DOI: 10.1101/cshperspect.a038448
Next-Generation Influenza Vaccines
Abstract
Most currently used conventional influenza vaccines are based on 1940s technology. Advances in vaccine immunogen design and delivery emerging over the last decade promise new options for improving influenza vaccines. In addition, new technologies for immune profiling provide better-defined immune correlates of protection and precise surrogate biomarkers for vaccine evaluations. Major technological advances include single-cell analysis, high-throughput antibody discovery, next-generation sequencing of antibody gene transcripts, antibody ontogeny, structure-guided immunogen design, nanoparticle display, delivery and formulation options, and better adjuvants. In this review, we provide our prospective outlook for improved influenza vaccines in the foreseeable future.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

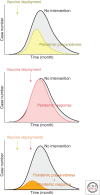
References
-
- Ahmed SS, Schur PH, MacDonald NE, Steinman L. 2014. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J Autoimmun 50: 1–11. 10.1016/j.jaut.2014.01.033 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
