Treatment outcome of anaplastic ependymoma under the age of 3 treated by intensity-modulated radiotherapy
- PMID: 32229806
- PMCID: PMC7113147
- DOI: 10.3857/roj.2020.00073
Treatment outcome of anaplastic ependymoma under the age of 3 treated by intensity-modulated radiotherapy
Abstract
Purpose: Intensity-modulated radiotherapy (IMRT) allows for more precise treatment, reducing unwanted radiation to nearby structures. We investigated the safety and feasibility of IMRT for anaplastic ependymoma patients below 3 years of age.
Materials and methods: A total of 9 anaplastic ependymoma patients below 3 years of age, who received IMRT between October 2011 and December 2017 were retrospectively reviewed. The median equivalent dose in 2 Gy fractions was 52.0 Gy (range, 48.0 to 60.0 Gy). Treatment outcomes and neurologic morbidities were reviewed in detail.
Results: The median patient age was 20.9 months (range, 12.1 to 31.2 months). All patients underwent surgery. The rates of 5-year overall survival, freedom from local recurrence, and progression-free survival were 40.6%, 53.3%, and 26.7%, respectively. Of the 9 patients, 5 experienced recurrences (3 had local recurrence, 1 had both local recurrence and cerebrospinal fluid [CSF] seeding, and 1 had CSF seeding alone). Five patients died because of disease progression. Assessment of neurologic morbidity revealed motor dysfunction in 3 patients, all of whom presented with hydrocephalus at initial diagnosis because of the location of the tumor and already had neurologic deficits before radiotherapy (RT).
Conclusion: Neurologic morbidity is not caused by RT alone but may result from mass effects of the tumor and surgical sequelae. Administration of IMRT to anaplastic ependymoma patients below 3 years of age yielded encouraging local control and tolerable morbidities. High-precision modern RT such as IMRT can be considered for very young patients with anaplastic ependymoma.
Keywords: Ependymoma; Intensity-modulated radiotherapy; Local neoplasm recurrence; Morbidity; Pediatrics.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
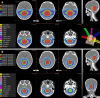


References
-
- Halperin EC, Brady LW, Perez CA, Wazer DE. Perez & Brady's principles and practice of radiation oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.
-
- White L, Kellie S, Gray E, et al. Postoperative chemotherapy in children less than 4 years of age with malignant brain tumors: promising initial response to a VETOPEC-based regimen. A Study of the Australian and New Zealand Children's Cancer Study Group (ANZCCSG) J Pediatr Hematol Oncol. 1998;20:125–30. - PubMed
-
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. - PubMed
LinkOut - more resources
Full Text Sources

