Detection of Rare Objects by Flow Cytometry: Imaging, Cell Sorting, and Deep Learning Approaches
- PMID: 32230871
- PMCID: PMC7177904
- DOI: 10.3390/ijms21072323
Detection of Rare Objects by Flow Cytometry: Imaging, Cell Sorting, and Deep Learning Approaches
Abstract
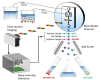
Flow cytometry nowadays is among the main working instruments in modern biology paving the way for clinics to provide early, quick, and reliable diagnostics of many blood-related diseases. The major problem for clinical applications is the detection of rare pathogenic objects in patient blood. These objects can be circulating tumor cells, very rare during the early stages of cancer development, various microorganisms and parasites in the blood during acute blood infections. All of these rare diagnostic objects can be detected and identified very rapidly to save a patient's life. This review outlines the main techniques of visualization of rare objects in the blood flow, methods for extraction of such objects from the blood flow for further investigations and new approaches to identify the objects automatically with the modern deep learning methods.
Keywords: cell labeling; cell sorting; circulating tumor cells; deep learning; flow cytometry data analysis; imaging flow cytometry; liquid biopsy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





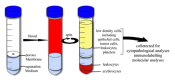

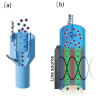


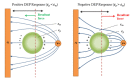






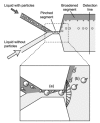
References
-
- Galanzha E.I., Menyaev Y.A., Yadem A.C., Sarimollaoglu M., Juratli M.A., Nedosekin D.A., Foster S.R., Jamshidi-Parsian A., Siegel E.R., Makhoul I., et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci. Transl. Med. 2019;11:eaat5857. doi: 10.1126/scitranslmed.aat5857. - DOI - PMC - PubMed
-
- Menyaev Y.A., Carey K.A., Nedosekin D.A., Sarimollaoglu M., Galanzha E.I., Stumhofer J.S., Zharov V.P. Preclinical photoacoustic models: Application for ultrasensitive single cell malaria diagnosis in large vein and artery. Biomed. Opt. Express. 2016;7:3643–3658. doi: 10.1364/BOE.7.003643. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

