Platinum(IV) Complexes of trans-1,2-diamino-4-cyclohexene: Prodrugs Affording an Oxaliplatin Analogue that Overcomes Cancer Resistance
- PMID: 32230896
- PMCID: PMC7177638
- DOI: 10.3390/ijms21072325
Platinum(IV) Complexes of trans-1,2-diamino-4-cyclohexene: Prodrugs Affording an Oxaliplatin Analogue that Overcomes Cancer Resistance
Abstract
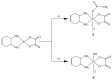
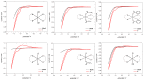
Six platinum(IV) compounds derived from an oxaliplatin analogue containing the unsaturated cyclic diamine trans-1,2-diamino-4-cyclohexene (DACHEX), in place of the 1,2-diaminocyclohexane, and a range of axial ligands, were synthesized and characterized. The derivatives with at least one axial chlorido ligand demonstrated solvent-assisted photoreduction. The electrochemical redox behavior was investigated by cyclic voltammetry; all compounds showed reduction potentials suitable for activation in vivo. X-ray photoelectron spectroscopy (XPS) data indicated an X-ray-induced surface reduction of the Pt(IV) substrates, which correlates with the reduction potentials measured by cyclic voltammetry. The cytotoxic activity was assessed in vitro on a panel of human cancer cell lines, also including oxaliplatin-resistant cancer cells, and compared with that of the reference compounds cisplatin and oxaliplatin; all IC50 values were remarkably lower than those elicited by cisplatin and somewhat lower than those of oxaliplatin. Compared to the other Pt(IV) compounds of the series, the bis-benzoate derivative was by far (5-8 times) the most cytotoxic showing that low reduction potential and high lipophilicity are essential for good cytotoxicity. Interestingly, all the complexes proved to be more active than cisplatin and oxaliplatin even in three-dimensional spheroids of A431 human cervical cancer cells.
Keywords: anticancer drugs; cisplatin; oxaliplatin; platinum(IV) prodrugs; trans-1,2-diamino-4-cyclohexene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

