Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources
- PMID: 32230973
- PMCID: PMC7180973
- DOI: 10.3390/molecules25071537
Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources
Abstract
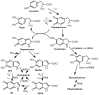
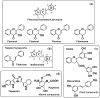
Direct sun exposure is one of the most aggressive factors for human skin. Sun radiation contains a range of the electromagnetic spectrum including UV light. In addition to the stratospheric ozone layer filtering the most harmful UVC, human skin contains a photoprotective pigment called melanin to protect from UVB, UVA, and blue visible light. This pigment is a redox UV-absorbing agent and functions as a shield to prevent direct UV action on the DNA of epidermal cells. In addition, melanin indirectly scavenges reactive oxygenated species (ROS) formed during the UV-inducing oxidative stress on the skin. The amounts of melanin in the skin depend on the phototype. In most phenotypes, endogenous melanin is not enough for full protection, especially in the summertime. Thus, photoprotective molecules should be added to commercial sunscreens. These molecules should show UV-absorbing capacity to complement the intrinsic photoprotection of the cutaneous natural pigment. This review deals with (a) the use of exogenous melanin or melanin-related compounds to mimic endogenous melanin and (b) the use of a number of natural compounds from plants and marine organisms that can act as UV filters and ROS scavengers. These agents have antioxidant properties, but this feature usually is associated to skin-lightening action. In contrast, good photoprotectors would be able to enhance natural cutaneous pigmentation. This review examines flavonoids, one of the main groups of these agents, as well as new promising compounds with other chemical structures recently obtained from marine organisms.
Keywords: antioxidant natural products; flavonoids.; melanin; photoprotection; sunscreen.
Conflict of interest statement
The author declares no conflicts of interest.
Figures



References
-
- Premi S., Wallisch S., Mano C.M., Weiner A.B., Bacchiocchi A., Wakamatsu K., Bechara E.J.H., Halaban R., Douki T., Brash D.E. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347:842–847. doi: 10.1126/science.1256022. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

