A randomized proof-of-mechanism trial applying the 'fast-fail' approach to evaluating κ-opioid antagonism as a treatment for anhedonia
- PMID: 32231295
- PMCID: PMC9949770
- DOI: 10.1038/s41591-020-0806-7
A randomized proof-of-mechanism trial applying the 'fast-fail' approach to evaluating κ-opioid antagonism as a treatment for anhedonia
Abstract
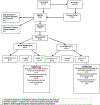
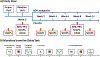
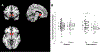
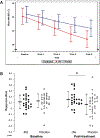
The National Institute of Mental Health (NIMH) 'fast-fail' approach seeks to improve too-often-misleading early-phase drug development methods by incorporating biomarker-based proof-of-mechanism (POM) testing in phase 2a. This first comprehensive application of the fast-fail approach evaluated the potential of κ-opioid receptor (KOR) antagonism for treating anhedonia with a POM study determining whether robust target engagement favorably impacts the brain circuitry hypothesized to mediate clinical effects. Here we report the results from a multicenter, 8-week, double-blind, placebo-controlled, randomized trial in patients with anhedonia and a mood or anxiety disorder (selective KOR antagonist (JNJ-67953964, 10 mg; n = 45) and placebo (n = 44)). JNJ-67953964 significantly increased functional magnetic resonance imaging (fMRI) ventral striatum activation during reward anticipation (primary outcome) as compared to placebo (baseline-adjusted mean: JNJ-67953964, 0.72 (s.d. = 0.67); placebo, 0.33 (s.d. = 0.68); F(1,86) = 5.58, P < 0.01; effect size = 0.58 (95% confidence interval, 0.13-0.99)). JNJ-67953964, generally well tolerated, was not associated with any serious adverse events. This study supports proceeding with assessment of the clinical impact of target engagement and serves as a model for implementing the 'fast-fail' approach.
Figures
Comment in
-
A precision medicine-based, 'fast-fail' approach for psychiatry.Nat Med. 2020 May;26(5):653-654. doi: 10.1038/s41591-020-0854-z. Nat Med. 2020. PMID: 32405056 No abstract available.
References
-
- Denys D, de Geus F. Predictors of pharmacotherapy response in anxiety disorders. Curr Psychiatry Rep 7(4), 252–7 (2005). - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163(11),1905–17 (2006). - PubMed
-
- Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv 60(11), 1466–7 (2009) - PubMed
-
- National Advisory Mental Health Workgroup. From Discovery to Cure: Accelerating the Development of New and Personalized Interventions for Mental Illness Washington, DC: NIMH. (2010).
-
- Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov 9(3), 203–214 (2010). - PubMed
METHODS ONLY REFERENCES
-
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders (4th ed., Text Revision). Washington, DC. (2009).
-
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baer R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20, 22–33 (1998). - PubMed
-
- Posner K Columbia-Suicide Severity Rating Scale (C-SSRS) http://www.cssrs.columbia.edu (2011).
-
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2), 825–41 (2002). - PubMed
-
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 5(2), 143–56 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

