Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer
- PMID: 32231310
- PMCID: PMC7194135
- DOI: 10.1038/s41556-020-0496-x
Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer
Abstract
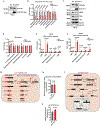
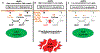
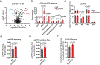
SLC7A11-mediated cystine uptake is critical for maintaining redox balance and cell survival. Here we show that this comes at a significant cost for cancer cells with high levels of SLC7A11. Actively importing cystine is potentially toxic due to its low solubility, forcing cancer cells with high levels of SLC7A11 (SLC7A11high) to constitutively reduce cystine to the more soluble cysteine. This presents a significant drain on the cellular NADPH pool and renders such cells dependent on the pentose phosphate pathway. Limiting glucose supply to SLC7A11high cancer cells results in marked accumulation of intracellular cystine, redox system collapse and rapid cell death, which can be rescued by treatments that prevent disulfide accumulation. We further show that inhibitors of glucose transporters selectively kill SLC7A11high cancer cells and suppress SLC7A11high tumour growth. Our results identify a coupling between SLC7A11-associated cystine metabolism and the pentose phosphate pathway, and uncover an accompanying metabolic vulnerability for therapeutic targeting in SLC7A11high cancers.
Conflict of interest statement
Competing Financial Interests
K.O. and M.V.P. are full-time employees of Kadmon Corporation, LLC. Other authors declare no competing financial interests.
Figures




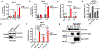






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

