Rosiglitazone Protects Endothelial Cells From Irradiation-Induced Mitochondrial Dysfunction
- PMID: 32231569
- PMCID: PMC7082323
- DOI: 10.3389/fphar.2020.00268
Rosiglitazone Protects Endothelial Cells From Irradiation-Induced Mitochondrial Dysfunction
Abstract
Background and purpose: Up to 50-60% of all cancer patients receive radiotherapy as part of their treatment strategy. However, the mechanisms accounting for increased vascular risks after irradiation are not completely understood. Mitochondrial dysfunction has been identified as a potential cause of radiation-induced atherosclerosis.
Materials and methods: Assays for apoptosis, cellular metabolism, mitochondrial DNA content, functionality and morphology were used to compare the response of endothelial cells to a single 2 Gy dose of X-rays under basal conditions or after pharmacological treatments that either reduced (EtBr) or increased (rosiglitazone) mitochondrial content.
Results: Exposure to ionizing radiation caused a persistent reduction in mitochondrial content of endothelial cells. Pharmacological reduction of mitochondrial DNA content rendered endothelial cells more vulnerable to radiation-induced apoptosis, whereas rosiglitazone treatment increased oxidative metabolism and redox state and decreased the levels of apoptosis after irradiation.
Conclusion: Pre-existing mitochondrial damage sensitizes endothelial cells to ionizing radiation-induced mitochondrial dysfunction. Rosiglitazone protects endothelial cells from the detrimental effects of radiation exposure on mitochondrial metabolism and oxidative stress. Thus, our findings indicate that rosiglitazone may have potential value as prophylactic for radiation-induced atherosclerosis.
Keywords: cardiovascular disease; endothelial cells; ionizing radiation; mitochondria; rosiglitazone.
Copyright © 2020 Baselet, Driesen, Coninx, Belmans, Sieprath, Lambrichts, De Vos, Baatout, Sonveaux and Aerts.
Figures
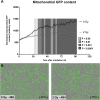
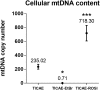


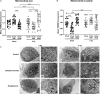
 ) exposure (N = 3, n = 32–64). *P < 0.05, ****P < 0.0001 by two-way ANOVA with Bonferroni post hoc test.
) exposure (N = 3, n = 32–64). *P < 0.05, ****P < 0.0001 by two-way ANOVA with Bonferroni post hoc test.
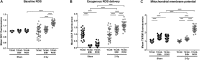
References
LinkOut - more resources
Full Text Sources

