Gastrointestinal toxic effects in patients with cancer receiving platinum-based therapy
- PMID: 32231718
- PMCID: PMC7097936
- DOI: 10.7150/jca.37777
Gastrointestinal toxic effects in patients with cancer receiving platinum-based therapy
Abstract
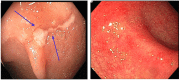
Background: Platinum-based therapy (PBT) can be limited by gastrointestinal adverse events, particularly PBT-related colitis and diarrhea (PCD). We studied clinical features, treatments, and outcomes of PCD. Methods: This was a retrospective study of cancer patients who received PBT and colonoscopic evaluation for PCD symptoms from 2009 to 2018. Results: Of 36,595 patients who received PBT, 86 (0.2%) met inclusion criteria. Median time from PBT initiation to PCD was 66 days. Regarding PBT type, 47% of the patients received carboplatin, 31% cisplatin, and 22% oxaliplatin. Median duration of PCD symptoms was 20 days. Colonoscopy revealed mucosal ulceration in 34% of the patients and nonulcerative inflammation in 33%. Half of the cohort needed hospitalization for PCD (49%). The majority received treatment for PCD (59%): immunosuppressive therapy in 21%, antibiotics in 27%, antimotility agents in 22%, and intravenous fluids in 51%. Eight patients (9%) were admitted to the intensive care unit for PCD management. Six patients (7%) experienced colonic perforation that required surgical intervention; two of them had gastrointestinal tumors. Physicians restarted PBT in 37 (43%) patients; 8 (22%) of them had PCD recurrence that was managed expectantly. Colonic perforation occurred more frequently with use of oxaliplatin and cisplatin than carboplatin (P=0.05). The median duration of PCD symptoms was longer in patients receiving carboplatin or cisplatin than in those receiving oxaliplatin (P=0.182). Conclusions: PCD is rare, but in a small subset of patients, it can lead to serious complications. Treatment of PCD is mainly supportive, but immunosuppressive therapy may be required.
Keywords: carboplatin; cisplatin; colitis; gastrointestinal toxicity; platinum.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Chen X, Y. Wu, H. Dong, et al. Platinum-based agents for individualized cancer treatment. Current molecular medicine. 2013;13(10):1603–12. - PubMed
-
- WHO Model Lists of Essential Medicines. Accessed online January 11, 2019. https://www.who.int/medicines/publications/essentialmedicines/en/
-
- Puisset F, A. Schmitt, and E. Chatelut, Standardization of chemotherapy and individual dosing of platinum compounds. Anticancer research. 2014;34(1):465–70. - PubMed
-
- Pfisterer J. and J.A. Ledermann, Management of platinum-sensitive recurrent ovarian cancer. Seminars in oncology. 2006;33(2 Suppl 6):S12–6. - PubMed
-
- McQuade R.M, V. Stojanovska, J.C. Bornstein, et al. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Current medicinal chemistry. 2017;24(15):1537–1557. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous