Autophagy Regulation by the Translation Machinery and Its Implications in Cancer
- PMID: 32232004
- PMCID: PMC7082396
- DOI: 10.3389/fonc.2020.00322
Autophagy Regulation by the Translation Machinery and Its Implications in Cancer
Abstract
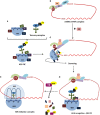
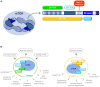
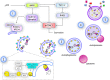
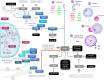
Various metabolic pathways and molecular processes in the cell act intertwined, and dysregulating the interplay between some of them may lead to cancer. It is only recently that defects in the translation process, i.e., the synthesis of proteins by the ribosome using a messenger (m)RNA as a template and translation factors, have begun to gain strong attention as a cause of autophagy dysregulation with effects in different maladies, including cancer. Autophagy is an evolutionarily conserved catabolic process that degrades cytoplasmic elements in lysosomes. It maintains cellular homeostasis and preserves cell viability under various stress conditions, which is crucial for all eukaryotic cells. In this review, we discuss recent advances shedding light on the crosstalk between the translation and the autophagy machineries and its impact on tumorigenesis. We also summarize how this interaction is being the target for novel therapies to treat cancer.
Keywords: ATG; PERK; autophagy; cancer; eIF2alpha; endoplasmic reticulum; mTOR; translation initiation.
Copyright © 2020 Acevo-Rodríguez, Maldonado, Castro-Obregón and Hernández.
Figures
References
-
- Hernández G, Tettweiler G. Protein abundance variation. In: Meyer RA, editor. Systems Biology. Weinheim: Wiley-Blackwell; (2012). p. 117–37. 10.1002/3527600906.mcb.201100039 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

