The Endosteal Niche in Breast Cancer Bone Metastasis
- PMID: 32232008
- PMCID: PMC7082928
- DOI: 10.3389/fonc.2020.00335
The Endosteal Niche in Breast Cancer Bone Metastasis
Abstract
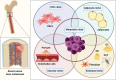
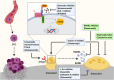
The establishment of bone metastasis remains one of the most frequent complications of patients suffering from advanced breast cancer. Patients with bone metastases experience high morbidity and mortality caused by excessive, tumor-induced and osteoclast-mediated bone resorption. Anti-resorptive treatments, such as bisphosphonates, are available to ease skeletal related events including pain, increased fracture risk, and hypercalcemia. However, the disease remains incurable and 5-year survival rates for these patients are below 25%. Within the bone, disseminated breast cancer cells localize in "metastatic niches," special microenvironments that are thought to regulate cancer cell colonization and dormancy as well as tumor progression and subsequent development into overt metastases. Precise location and composition of this "metastatic niche" remain poorly defined. However, it is thought to include an "endosteal niche" that is composed of key bone cells that are derived from both, hematopoietic stem cells (osteoclasts), and mesenchymal stromal cells (osteoblasts, fibroblasts, adipocytes). Our knowledge of how osteoclasts drive the late stage of the disease is well-established. In contrast, much less is known about the interaction between osteogenic cells and disseminated tumor cells prior to the initiation of the osteolytic phase. Recent studies suggest that mesenchymal-derived cells, including osteoblasts and fibroblasts, play a key role during the early stages of breast cancer bone metastasis such as tumor cell homing, bone marrow colonization, and tumor cell dormancy. Hence, elucidating the interactions between breast cancer cells and mesenchymal-derived cells that drive metastasis progression could provide novel therapeutic approaches and targets to treat breast cancer bone metastasis. In this review we discuss evidences reporting the interaction between tumor cells and endosteal niche cells during the early stages of breast cancer bone metastasis, with a particular focus on mesenchymal-derived osteoblasts and fibroblasts.
Keywords: bone metastases; breast cancer; endosteal niche; fibroblast; microenvironment; osteoblast.
Copyright © 2020 Haider, Smit and Taipaleenmäki.
Figures

References
-
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. (1989) 8:98–101. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

