Prospective trial examining safety and efficacy of microcurrent stimulation for the treatment of sinus pain and congestion
- PMID: 32232107
- PMCID: PMC7098235
- DOI: 10.1186/s42234-019-0035-x
Prospective trial examining safety and efficacy of microcurrent stimulation for the treatment of sinus pain and congestion
Erratum in
-
Correction to: Prospective trial examining safety and efficacy of microcurrent stimulation for the treatment of sinus pain and congestion.Bioelectron Med. 2020 Jan 30;6:2. doi: 10.1186/s42234-020-0039-6. eCollection 2020. Bioelectron Med. 2020. PMID: 32232222 Free PMC article.
Abstract
Background: Inflammation and swelling of the sinus and nasal mucosa are commonly caused by viral infection, bacterial infection, or exposure to allergens and irritants. Sinonasal inflammation can cause symptoms of nasal congestion, facial pressure, and rhinogenic facial pain or "sinus pain". A previous randomized controlled study demonstrated that acute treatment with non-invasive periorbital microcurrent stimulation resulted in a rapid and clinically meaningful reduction in self-report of sinus pain that significantly outperformed sham control treatment. Here, we assessed the acute durability of microcurrent pain relief and longitudinal effects of 4 weeks of daily microcurrent treatment in patients presenting with sinus pain.
Methods: Thirty subjects with moderate facial pain (numeric rating scale ≥5) attributed to self-reported sinonasal disease were enrolled in a single-arm, prospective interventional study. At enrollment, subjects were given a microcurrent treatment device and written instructions and self-administered the device to the bilateral periorbital regions for 5 mins. Subjects were instructed to treat themselves at home once daily and up to four times daily as needed for 4 weeks. Pain was measured both acutely and weekly during the 4 weeks of treatment using the numeric rating scale. Congestion and medication use data were collected weekly using the Congestion Quantifier 7 (CQ7) and medication diary, respectively.
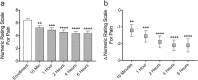
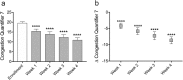
Results: Thirty patients were enrolled and completed the study. Microcurrent therapy rapidly reduced post-treatment numeric rating scale for pain by - 1.2 at 10 mins (p = 0.0076), - 1.6 at 1 hr (p = 0.0007), - 1.9 at 2 hrs (p < 0.0001), - 2.1 at 4 hrs (p < 0.0001), and - 2.1 at 6 hrs (p < 0.0001). With daily microcurrent treatment, numeric rating scale for pain was reduced over 4 weeks by - 1.3 (- 20.1%) after 1 week (p = 0.0018), - 2.1 (- 32.1%) after 2 weeks (p < 0.0001), - 2.4 (- 36.6%) after 3 weeks (p < 0.0001) and - 2.9 (- 43.3%) after 4 weeks (p < 0.0001). For subjects who enrolled with moderate or worse congestion, mean congestion scores (CQ7) were reduced by - 4.2 (- 22.0%) after 1 week (p < 0.0001), - 5.8 (- 33.0%) after 2 weeks (p < 0.0001), - 7.2 (- 37.4%) after 3 weeks (p < 0.0001) and - 8.6 (- 44.3%) after 4 weeks (p < 0.0001) of microcurrent treatment.
Conclusion: Self-administered periorbital microcurrent treatment given at home was efficacious in significantly reducing moderate sinus pain for up to 6 hrs and significantly reducing moderate pain and congestion over 4 weeks of daily use. Microcurrent therapy was found to be safe with only minor side effects that resolved without intervention.
Trial registration: ClinicalTrials.gov, NCT03888274. Registered 25 March 2019. Retroactively registered, https://clinicaltrials.gov/ct2/show/NCT03888274.
Keywords: Allergic rhinitis; Congestion; Cranial nerve; Facial pain; Microcurrent; Nasal congestion; Rhinologic facial pain; Sinus pain; Transcutaneous electrical nerve stimulation; Trigeminal nerve.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsA.B.G. is a member of the medical advisory board for Tivic Health Systems, Inc.; N.P. is a former contractor for Tivic Health Systems, Inc.; B.T.G is an employee of Tivic Health Systems Inc.
Figures



References
-
- Blackwell DL, Villarroel MA, T.C. C. Tables of summary health statistics for U.S. adults: 2013 National Health Interview Survey.: Atlanta: Centers for Disease Control and Prevention; 2015 [Available from: https://www.cdc.gov/nchs/nhis/SHS/tables.htm.
-
- Fischer L, Auberson S, Bretton C, Lacroix JS. Adrenergic and non-adrenergic vasoconstrictor mechanisms in the human nasal mucosa. Rhinology. 1993;31(1):11–15. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
