Irradiated tumor cell-derived microparticles mediate tumor eradication via cell killing and immune reprogramming
- PMID: 32232155
- PMCID: PMC7096163
- DOI: 10.1126/sciadv.aay9789
Irradiated tumor cell-derived microparticles mediate tumor eradication via cell killing and immune reprogramming
Abstract
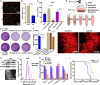
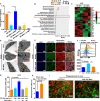
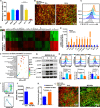
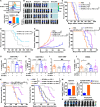
Radiotherapy (RT) is routinely used in cancer treatment, but expansion of its clinical indications remains challenging. The mechanism underlying the radiation-induced bystander effect (RIBE) is not understood and not therapeutically exploited. We suggest that the RIBE is predominantly mediated by irradiated tumor cell-released microparticles (RT-MPs), which induce broad antitumor effects and cause immunogenic death mainly through ferroptosis. Using a mouse model of malignant pleural effusion (MPE), we demonstrated that RT-MPs polarized microenvironmental M2 tumor-associated macrophages (M2-TAMs) to M1-TAMs and modulated antitumor interactions between TAMs and tumor cells. Following internalization of RT-MPs, TAMs displayed increased programmed cell death ligand 1 (PD-L1) expression, enhancing follow-up combined anti-PD-1 therapy that confers an ablative effect against MPE and cisplatin-resistant MPE mouse models. Immunological memory effects were induced.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures
References
-
- Ethun C. G., Bilen M. A., Jani A. B., Maithel S. K., Ogan K., Master V. A., Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 67, 362–377 (2017). - PubMed
-
- Herrera F. G., Bourhis J., Coukos G., Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 67, 65–85 (2017). - PubMed
-
- Joyce J. A., Fearon D. T., T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

