Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 Clinical Trial
- PMID: 32232481
- PMCID: PMC7316220
- DOI: 10.1182/blood.2019003277
Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 Clinical Trial
Abstract
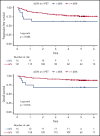
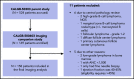
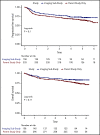
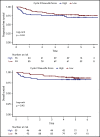
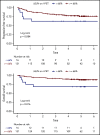
As part of a randomized, prospective clinical trial in large cell lymphoma, we conducted serial fluorodeoxyglucose positron emission tomography (FDG-PET) at baseline, after 2 cycles of chemotherapy (interim PET [i-PET]), and at end of treatment (EoT) to identify biomarkers of response that are predictive of remission and survival. Scans were interpreted in a core laboratory by 2 imaging experts, using the visual Deauville 5-point scale (5-PS), and by calculating percent change in FDG uptake (change in standardized uptake value [ΔSUV]). Visual scores of 1 through 3 and ΔSUV ≥66% were prospectively defined as negative. Of 524 patients enrolled in the parent trial, 169 agreed to enroll in the PET substudy and 158 were eligible for final analysis. In this selected population, all had FDG-avid disease at baseline; by 5-PS, 55 (35%) remained positive on i-PET and 28 (18%) on EoT PET. Median ΔSUV on i-PET was 86.2%. With a median follow-up of 5 years, ΔSUV, as continuous variable, was associated with progression-free survival (PFS) (hazard ratio [HR] = 0.99; 95% confidence interval [CI], 0.97-1.00; P = .02) and overall survival (OS) (HR, 0.98; 95% CI, 0.97-0.99; P = .03). ΔSUV ≥66% was predictive of OS (HR, 0.31; 95% CI, 0.11-0.85; P = .02) but not PFS (HR, 0.47; 95% CI, 0.19-1.13; P = .09). Visual 5-PS on i-PET did not predict outcome. ΔSUV, but not visual analysis, on i-PET predicted OS in DLBCL, although the low number of events limited the statistical analysis. These data may help guide future clinical trials using PET response-adapted therapy. This trial was registered at www.clinicaltrials.gov as #NCT00118209.
Conflict of interest statement
Conflict-of-interest disclosure: H.S. was a consultant to Aileron Therapeutics until June 30, 2018 (unrelated to current work). A.D.Z. serves or has served as a consultant for Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnology, and Verastem; he serves on the advisory board of MorphoSys, Gilead, Genentech, Abbvie, and AstraZeneca Pharmacyclics and receives research support from MEI Pharmaceuticals, Roche, Gilead, and Beigene; he also serves as the DMC Chair for Beigene. N.W.-J. serves or has served on the advisory boards for Bayer, Gilead, ADC Therapeutics, and Janssen. B.K. serves as a consultant for Genentech and Roche, and receives research funding from Genentech. J.F. has received honoraria from Bayer and Ascerta for data and safety monitoring committee activities. E.H. receives research support from Eli Lilly & Co. and Abbvie and serves on the honoraria advisory boards of Seattle Genetics, Celgene, and Jazz Pharmaceuticals. J.P.L. serves or has served as a consultant for Sutro, Bayer, Gilead, AstraZeneca, Celgene, Roche/Genentech, ADC Therapeutics, Sandoz, Karyopharm, Miltenyi, Novartis, Biotest, Merck, Morphosys, Beigene, Nordic Nanovector, BMS, Akcea Therapeutics, Epizyme, and MEI Pharma. L.H.S. has received third-party payments from Merck, Roche, and Pfizer for participating on data safety monitoring and endpoint committees and has served as a consultant for Boehringer and Imaging Endpoints. The remaining authors declare no competing financial interests.
Figures

Comment in
-
ΔSUVmax for interim PET in DLBCL: old is new.Blood. 2020 Jun 18;135(25):2202-2203. doi: 10.1182/blood.2020005649. Blood. 2020. PMID: 32556130 No abstract available.
References
-
- Johnson SA, Kumar A, Matasar MJ, Schöder H, Rademaker J. Imaging for staging and response assessment in lymphoma. Radiology. 2015;276(2):323-338. - PubMed
-
- Moskowitz CH, Schöder H. Current status of the role of PET imaging in diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):138-142. - PubMed
-
- Gallamini A, Zwarthoed C. Interim FDG-PET imaging in lymphoma. Semin Nucl Med. 2018;48(1):17-27. - PubMed
-
- Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the first international workshop on interim-PET-scan in lymphoma. Leuk Lymphoma. 2009;50(8):1257-1260. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180821/CA/NCI NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA189960/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

