Wolff-Parkinson-White syndrome: De novo variants and evidence for mutational burden in genes associated with atrial fibrillation
- PMID: 32233023
- PMCID: PMC7275694
- DOI: 10.1002/ajmg.a.61571
Wolff-Parkinson-White syndrome: De novo variants and evidence for mutational burden in genes associated with atrial fibrillation
Abstract
Background: Wolff-Parkinson-White (WPW) syndrome is a relatively common arrhythmia affecting ~1-3/1,000 individuals. Mutations in PRKAG2 have been described in rare patients in association with cardiomyopathy. However, the genetic basis of WPW in individuals with a structurally normal heart remains poorly understood. Sudden death due to atrial fibrillation (AF) can also occur in these individuals. Several studies have indicated that despite ablation of an accessory pathway, the risk of AF remains high in patients compared to general population.
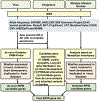
Methods: We applied exome sequencing in 305 subjects, including 65 trios, 80 singletons, and 6 multiple affected families. We used de novo analysis, candidate gene approach, and burden testing to explore the genetic contributions to WPW.
Results: A heterozygous deleterious variant in PRKAG2 was identified in one subject, accounting for 0.6% (1/151) of the genetic basis of WPW in this study. Another individual with WPW and left ventricular hypertrophy carried a known pathogenic variant in MYH7. We found rare de novo variants in genes associated with arrhythmia and cardiomyopathy (ANK2, NEBL, PITX2, and PRDM16) in this cohort. There was an increased burden of rare deleterious variants (MAF ≤ 0.005) with CADD score ≥ 25 in genes linked to AF in cases compared to controls (P = .0023).
Conclusions: Our findings show an increased burden of rare deleterious variants in genes linked to AF in WPW syndrome, suggesting that genetic factors that determine the development of accessory pathways may be linked to an increased susceptibility of atrial muscle to AF in a subset of patients.
Keywords: ANK2; Wolff-Parkinson-White (WPW) syndrome; atrial fibrillation; exome sequencing.
© 2020 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
Baylor College of Medicine (BCM) and Miraca Holdings Inc. have formed a joint venture with shared ownership and governance of Baylor Genetics (BG), formerly the Baylor Miraca Genetics Laboratories (BMGL), which performs clinical exome sequencing. J.R.L. serves on the Scientific Advisory Board of BG. J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals, and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases and bacterial genomic fingerprinting. J.W.B. contributed to this work while employed by BCM and is currently a full-time employee of Illumina, Inc. Other authors have no disclosures relevant to the manuscript.
Figures

References
-
- Arad M, Moskowitz IP, Patel VV, Ahmad F, Perez-Atayde AR, Sawyer DB, …Seidman JG (2003). Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation, 107(22), 2850–6. doi:10.1161/01.CIR.0000075270.13497.2B 01.CIR.0000075270.13497.2B [pii] - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

