Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity
- PMID: 32233024
- PMCID: PMC7189993
- DOI: 10.1111/acel.13142
Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity
Abstract
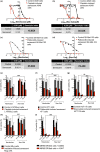
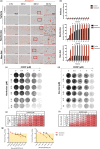
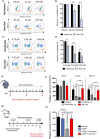
Pharmacologically active compounds with preferential cytotoxic activity for senescent cells, known as senolytics, can ameliorate or even revert pathological manifestations of senescence in numerous preclinical mouse disease models, including cancer models. However, translation of senolytic therapies to human disease is hampered by their suboptimal specificity for senescent cells and important toxicities that narrow their therapeutic windows. We have previously shown that the high levels of senescence-associated lysosomal β-galactosidase (SA-β-gal) found within senescent cells can be exploited to specifically release tracers and cytotoxic cargoes from galactose-encapsulated nanoparticles within these cells. Here, we show that galacto-conjugation of the BCL-2 family inhibitor Navitoclax results in a potent senolytic prodrug (Nav-Gal), that can be preferentially activated by SA-β-gal activity in a wide range of cell types. Nav-Gal selectively induces senescent cell apoptosis and has a higher senolytic index than Navitoclax (through reduced activation in nonsenescent cells). Nav-Gal enhances the cytotoxicity of standard senescence-inducing chemotherapy (cisplatin) in human A549 lung cancer cells. Concomitant treatment with cisplatin and Nav-Gal in vivo results in the eradication of senescent lung cancer cells and significantly reduces tumour growth. Importantly, galacto-conjugation reduces Navitoclax-induced platelet apoptosis in human and murine blood samples treated ex vivo, and thrombocytopenia at therapeutically effective concentrations in murine lung cancer models. Taken together, we provide a potentially versatile strategy for generating effective senolytic prodrugs with reduced toxicities.
Keywords: Navitoclax (ABT-263); cellular senescence; chemotherapy-induced senescence; lung cancer; prodrug; senolytics; thrombocytopenia.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
R.M.‐M. and M.S. are founders and advisors of Senolytic Therapeutics, Inc. C.P.M is an AstraZeneca employee. The remaining authors declare no competing interests.
Figures



References
-
- Agostini, A. , Mondragõn, L. , Bernardos, A. , Martínez‐Máñez, R. , Dolores Marcos, M. , Sancenõn, F. , … Murguía, J. R. (2012). Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angewandte Chemie (International Ed. in English), 51, 10556–10560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

