Short-term echocardiographic evaluation by global longitudinal strain in patients with heart failure treated with sacubitril/valsartan
- PMID: 32233080
- PMCID: PMC7261528
- DOI: 10.1002/ehf2.12656
Short-term echocardiographic evaluation by global longitudinal strain in patients with heart failure treated with sacubitril/valsartan
Abstract
Aims: The angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan reduces mortality and hospitalizations in patients with heart failure and reduced ejection fraction (HFrEF). Favourable effects on haemodynamic and functional parameters have been observed in patients with HFrEF undergoing ARNI therapy, using standard transthoracic echocardiography. Global longitudinal strain (GLS) assessment uses a semi-automatic procedure to provide a reliable and repeatable method that improves the detection of early changes of contractile function. We aimed to assess the effects of ARNI on GLS and myocardial mechanics in patients with HFrEF.
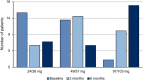
Methods and results: Thirty patients with New York Heart Association class II-III HFrEF were treated with ARNI and monitored using standard echocardiographic examination and GLS measurements at baseline, 3 months, and 6 months. ARNI therapy resulted in a significant reduction of ventricular volumes and a significant increase in left ventricular ejection fraction at 6 months but not 3 months by standard transthoracic echocardiography (left ventricular ejection fraction from 28 ± 8% at baseline to 34 ± 12% at 6 months, P < 0.001). Non-significant differences in the size of the left atrium, right ventricular function, and pulmonary pressures were found at 6 months. By using GLS, there was a progressive improvement of all strain parameters by 3 months. The improvement showed a progressive trend over time and maintained significance at 6 months: GLS 4ch -7.2 ± 4.8% at baseline vs. -7.5 ± 3.9% at 3 months (P = 0.025) and - 9.2 ± 5.2% at 6 months (P = 0.0001); AVG GLS -6.9 ± 4.3 at baseline vs. -7.9 ± 4.2 at 3 months (P = 0.04) and - 8.8 ± 4.4 at 6 months (P = 0.035); GLS endo 8.2 ± 4.8 at baseline vs. -9.0 ± 4.8 at 3 months (P = 0.05) and - 10.1 ± 5.1 at 6 months (P = 0.001).
Conclusions: Sacubitril/valsartan induces an early benefit on left ventricular remodelling, which is captured by myocardial strain and not by standard echocardiography. Strain method represents a practical tool to assess early and minimal variations of left ventricular systolic function.
Keywords: Angiotensin receptor neprilysin inhibitor; Global longitudinal strain; Heart failure; Heart failure with reduced ejection fraction; Neprilysin; Renin-angiotensin-aldosterone system.
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Conflict of interest statement
None declared.
Figures


References
-
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. - PubMed
-
- Xie W, Zheng F, Song X, Zhong B, Yan L. Renin‐angiotensin‐aldosterone system blockers for heart failure with reduced ejection fraction or left ventricular dysfunction: network meta‐analysis. Int J Cardiol 2016; 205: 65–71. - PubMed
-
- Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD, PROVE‐HF Investigators . Association of change in N‐terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019:1–11. 10.1001/jama.2019.12821 - DOI - PMC - PubMed
-
- Kapelios CJ, Lainscak M, Savarese G, Laroche C, Seferovic P, Ruschitzka F, Coats A, Anker SD, Crespo‐Leiro MG, Filippatos G, Piepoli MF, Rosano G, Zanolla L, Aguiar C, Murin J, Leszek P, McDonagh T , Maggioni AP, Lund LH, Heart Failure Long‐Term Registry Investigators . Sacubitril/valsartan eligibility and outcomes in the ESC‐EORP‐HFA Heart Failure Long‐Term Registry: bridging between European Medicines Agency/Food and Drug Administration label, the PARADIGM‐HF trial, ESC guidelines, and real world. Eur J Heart Fail 2019;21:1383–1397. - PubMed
-
- Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, Investigators PIONEER‐HF. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019; 380: 539–548. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

