Cortical Neural Stem Cell Lineage Progression Is Regulated by Extrinsic Signaling Molecule Sonic Hedgehog
- PMID: 32234482
- PMCID: PMC7197103
- DOI: 10.1016/j.celrep.2020.03.027
Cortical Neural Stem Cell Lineage Progression Is Regulated by Extrinsic Signaling Molecule Sonic Hedgehog
Abstract
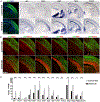
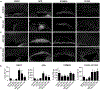
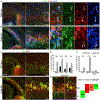
Neural stem cells (NSCs) in the prenatal neocortex progressively generate different subtypes of glutamatergic projection neurons. Following that, NSCs have a major switch in their progenitor properties and produce γ-aminobutyric acid (GABAergic) interneurons for the olfactory bulb (OB), cortical oligodendrocytes, and astrocytes. Herein, we provide evidence for the molecular mechanism that underlies this switch in the state of neocortical NSCs. We show that, at around E16.5, mouse neocortical NSCs start to generate GSX2-expressing (GSX2+) intermediate progenitor cells (IPCs). In vivo lineage-tracing study revealed that GSX2+ IPC population gives rise not only to OB interneurons but also to cortical oligodendrocytes and astrocytes, suggesting that they are a tri-potential population. We demonstrated that Sonic hedgehog signaling is both necessary and sufficient for the generation of GSX2+ IPCs by reducing GLI3R protein levels. Using single-cell RNA sequencing, we identify the transcriptional profile of GSX2+ IPCs and the process of the lineage switch of cortical NSCs.
Keywords: Gli3; Gsx2; Shh; cerebral cortex; neural stem cells; olfactory bulb interneurons; oligodendrocytes.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests J.L.R. and A.R.K. are cofounders, stockholders, and on the scientific board of Neurona, a company studying the potential therapeutic use of interneuron transplantation. The other authors declare no competing interests.
Figures




References
-
- Anderson SA, Eisenstat DD, Shi L, and Rubenstein JL (1997). Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476. - PubMed
-
- Blondel VD, Guillaume J-L, Lambiotte R, and Lefebvre E (2008). Fast unfolding of communities in large networks. J. Stat. Mech 10, P10008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

